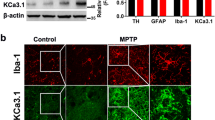
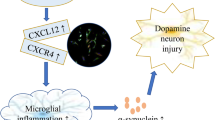
Abstract
Abundant reactive gliosis and neuroinflammation are typical pathogenetic hallmarks of brains in Parkinson’s disease (PD) patients, but regulation mechanisms are poorly understood. We are interested in role of programmed death-1 (PD-1) in glial reaction, neuroinflammation and neuronal injury in PD pathogenesis. Using PD mouse model and PD-1 knockout (KO) mice, we designed wild-type-control (WT-CON), WT-1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (WT-MPTP), PD-1-KO-control (KO-CON) and PD-1-KO-MPTP (KO-MPTP), and observed motor dysfunction of animal, morphological distribution of PD-1-positive cells, dopaminergic neuronal injury, glial activation and generation of inflammatory cytokines in midbrains by motor behavior detection, immunohistochemistry and western blot. WT-MPTP mouse model exhibited decrease of PD-1/Iba1-positive microglial cells in the substantia nigra compared with WT-CON mice. By comparison of four groups, PD-1 deficiency showed exacerbation in motor dysfunction of animals, decreased expression of TH protein and TH-positive neuronal protrusions. PD-1 deficiency enhanced microglial activation, production of proinflammatory cytokines like inducible nitric oxide synthase, tumor necrosis factor-α, interleukin-1β and interleukin-6, and expression and phosphorylation of AKT and ERK1/2 in the substantia nigra of MPTP model. We concluded that PD-1 deficiency could aggravate motor dysfunction of MPTP mouse model by inducing microglial activation and neuroinflammation in midbrains, suggesting that PD-1 signaling abnormality might be possibly involved in PD pathogenesis.








Similar content being viewed by others
Availability of data and materials
Data and materials from this manuscript are available upon request.
Abbreviations
- AD:
-
Alzheimer’s disease
- ARG1:
-
Arginase 1
- GFAP:
-
Glial fibrillary acidic protein
- IFNγ:
-
Interferon-γ
- IL-6:
-
Interleukin-6
- IL-10:
-
Interleukin-10
- iNOS:
-
Inducible nitric oxide synthase
- MPTP:
-
1-Methyl-4-phenyl-1,2,3,6-tetrahydropyridine
- PD:
-
Parkinson’s disease
- PD-1:
-
Programmed death receptor-1
- PD-L1:
-
Programmed death ligand 1
- TGF-β:
-
Transforming growth factor-beta
- TNF-α:
-
Tumor necrosis factor-alpha
References
Armstrong MJ, Okun MS (2020) Diagnosis and treatment of Parkinson disease: A review. JAMA 323:548–560
Azam S, Haque ME, Kim IS, Choi DK (2021) Microglial Turnover in ageing-related neurodegeneration: therapeutic avenue to intervene in disease progression. Cells 10:150
Poewe W, Seppi K, Tanner CM, Halliday GM, Brundin P, Volkmann J, Schrag AE, Lang AE (2017) Parkinson disease Nat Rev Dis Primers 3:17013
Muzio L, Viotti A, Martino G (2021) Microglia in Neuroinflammation and Neurodegeneration: From Understanding to Therapy. Front Neurosci 15:742065
Chetia Phukan B, Dutta A, Deb S, Saikia R, Mazumder MK, Paul R, Bhattacharya P, Sandhir R, Borah A (2021) Garcinol blocks motor behavioural deficits by providing dopaminergic neuroprotection in MPTP mouse model of Parkinson's disease: involvement of anti-inflammatory response. Exp Brain Res 2021 (Epub ahead of print).
Izco M, Blesa J, Verona G, Cooper JM, Alvarez-Erviti L (2021) Glial activation precedes alpha-synuclein pathology in a mouse model of Parkinson’s disease. Neurosci Res 170:330–340
Bernaus A, Blanco S, Sevilla A (2020) Glia crosstalk in neuroinflammatory diseases. Front Cell Neurosci 14:209
Rocha NP, Miranda ASd, Teixeira AL (2015) Insights into neuroinflammation in Parkinson’s disease: from biomarkers to anti-inflammatory based therapies. Biomed Res Int 2015:628192
Masuda T, Sankowski R, Staszewski O, Prinz M (2020) Microglia heterogeneity in the single-cell era. Cell Rep 30:1271–1281
Madore C, Yin ZR, Leibowitz J, Butovsky O (2020) Microglia, lifestyle stress, and neurodegeneration. Immunity 52:222–240
Khakh BS, Deneen B (2019) The emerging nature of astrocyte diversity. Annu Rev Neurosci 42:187–207
Grigoriadis N, Pesch VV (2015) A basic overview of multiple sclerosis immunopathology. Eur J Neurol 22(Suppl 2):3–13
Vivekanantham S, Shah S, Dewji R, Dewji A, Khatri C, Ologunde R (2015) Neuroinflammation in Parkinson’s disease: role in neurodegeneration and tissue repair. Int J Neurosci 125:717–725
Sochocka M, Diniz BS, Leszek J (2017) Inflammatory response in the CNS: Friend or Foe? Mol Neurobiol 54:8071–8089
Heneka MT, Kummer MP, Latz E (2014) Innate immune activation in neurodegenerative disease. Nat Rev Immunol 14:463–477
Ishida Y, Agata Y, Shibahara K, Honjo T (1992) Induced expression of PD-1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. EMBO J 11:3887–3895
Sun C, Mezzadra R, Schumacher TN (2018) Regulation and function of the PD-L1 checkpoint. Immunity 48:434–452
Balar AV, Weber JS (2017) PD-1 and PD-L1 antibodies in cancer: current status and future directions. Cancer Immunol Immunother 66:551–564
Ferris R (2013) PD-1 targeting in cancer immunotherapy. Cancer 119:E1-3
Saresella M, Calabrese E, Marventano I, Piancone F, Gatti A, Farina E, Alberoni M, Clerici M (2012) A potential role for the PD1/PD-L1 pathway in the neuroinflammation of Alzheimer’s disease. Neurobiol Aging 33:624.e11–22
Li PY, Mao LL, Liu XR, Gan Y, Zheng J, Thomson AW, Gao YQ, Chen J, Hu XM (2014) Essential role of program death 1-ligand 1 in regulatory T-cell-afforded protection against blood-brain barrier damage after stroke. Stroke 45:857–864
Javan MR, Aslani S, Zamani MR, Rostamnejad J, Asadi M, Farhoodi M, Nicknam MH (2016) Downregulation of immunosuppressive molecules, PD-1and PD-L1 but not PD-L2, in the patients with multiple sclerosis. Iran J Allergy Asthma Immunol 15:296–302
Han RR, Luo JY, Shi YC, Yao Y, Hao JW (2017) PD-L1 (Programmed death ligand 1) protects against experimental intracerebral hemorrhage-induced brain injury. Stroke 48:2255–2262
Chen Q, Xu LX, Du TJ, Hou YX, Fan WJ, Wu QL, Yan H (2019) Enhanced expression of PD-L1 on microglia after surgical brain injury exerts self-protection from inflammation and promotes neurological repair. Neurochem Res 44:2470–2481
Chauhan P, Lokensgard JR (2019) Glial cell expression of PD-L1. Int J Mol Sci 20:1677
Carter LL, Leach MW, Azoitei ML, Cui JQ, Pelker JW, Jussif J, Benoit S, Ireland G, Luxenberg D, Askew GR, Milarski KL, Groves C, Brown T, Carito BA, Percival K, Carreno BM, Collins M, Marusic S (2007) PD-1/PD-L1, but not PD-1/PD-L2, interactions regulate the severity of experimental autoimmune encephalomyelitis. J Neuroimmunol 182:124–134
Salama AD, Chitnis T, Imitola J, Akiba H, Tushima F, Azuma M, Yagita H, Sayegh MH, Khoury SJ (2003) Critical role of the programmed death-1 (PD-1) pathway in regulation of experimental autoimmune encephalomyelitis. J Exp Med 198:71–78
Zhu B, Guleria I, Khosroshahi A, Tanuja C, Imitola J, Azuma M, Yagita H, Sayegh MH, Khoury SJ (2006) Differential role of programmed death-ligand 1 and programmed death-ligand 2 in regulating the susceptibility and chronic progression of experimental autoimmune encephalomyelitis. J Immunol 176:3480–3489
Yao AH, Liu FF, Chen K, Tang L, Liu L, Zhang K, Yu CY, Bian GL, Guo HM, Zheng JJ, Cheng P, Ju G, Wang J (2014) Programmed death 1 deficiency induces the polarization of macrophages/microglia to the M1 phenotype after spinal cord injury in mice. Neurotherapeutics 11:636–650
Chen LW, Wei LC, Qiu Y, Liu HL, Rao ZR, Ju G, Chan YS (2002) Significant up-regulation of nestin protein in the neostriatum of MPTP-treated mice: Are the striatal astrocytes regionally activated after systemic MPTP administration? Brain Res 925:9–17
Bian GL, Wei LC, Shi M, Wang YQ, Cao R, Chen LW (2007) Fluoro-Jade C can specifically stain the degenerative neurons in the substantia nigra of the 1-methy-4- phenyl-1,2,3,6- tetrahydropyrindine-treated C57BL/6 mice. Brain Res 1150:55–61
Ding YX, Xia Y, Jiao XY, Duan L, Yu J, Wang X, Chen LW (2011) The TrkB-positive dopaminergic neurons are less sensitive to MPTP insult in the substantia nigra of adult C57/BL mice. Neurochem Res 36:1759–1766
Cheng YY, Ding YX, Bian GL, Chen LW, Yao XY, Lin YB, Wang Z, Chen BY (2020) Reactive astrocytes display pro-inflammatory adaptability with modulation of Notch-PI3K-AKT signaling pathway under inflammatory stimulation. Neuroscience 440:130–145
Rothhammer V, Quintana FJ (2015) Control of autoimmune CNS inflammation by astrocytes. Semin Immunopathol 37:625–638
Xu JW, Dong HQ, Qian QQ, Zhang X, Wang YW, Jin WJ, Qian YN (2017) Astrocyte-derived CCL2 participates in surgery-induced cognitive dysfunction and neuroinflammation via evoking microglia activation. Behav Brain Res 332:145–153
Waisman A, Liblau RS, Becher B (2015) Innate and adaptive immune responses in the CNS. Lancet Neurol 14:945–955
Andrieua GP, Shafrana JS, Smithd CL, Belkina AC, Casey AN, Jafari N, Denis GV (2019) BET protein targeting suppresses the PD-1/PD-L1 pathway in triple-negative breast cancer and elicits anti-tumor immune response. Cancer Lett 465:45–58
Ansari MJ, Salama AD, Chitnis T, Smith RN, Yagita H, Akiba H, Yamazaki T, Azuma M, Iwai H, Khoury SJ Jr, HA, Sayegh MH, (2003) The programmed death-1 (PD-1) pathway regulates autoimmune diabetes in nonobese diabetic (NOD) mice. J Exp Med 198:63–69
Wu J, Sun L, Li HY, Shen HT, Zhai WW, Yu ZQ, Chen G (2017) Roles of programmed death protein 1/programmed death-ligand 1 in secondary brain injury after intracerebral hemorrhage in rats: selective modulation of microglia polarization to anti-inflammatory phenotype. J Neuroinflammation 14:36
Villar-Cheda B, Dominguez-Meijide A, Joglar B, Rodriguez-Perez AI, Guerra MJ, Labandeira-Garcia JL (2012) Involvement of microglial RhoA/Rho-kinase pathway activation in the dopaminergic neuron death. Role of angiotensin via angiotensin type 1 receptors. Neurobiol Dis 47:268–279
Borrajo A, Rodriguez-Perez AI, Villar-Cheda B, Guerra MJ, Labandeira-Garcia JL (2014) Inhibition of the microglial response is essential for the neuroprotective effects of Rho-kinase inhibitors on MPTP-induced dopaminergic cell death. Neuropharmacology 85:1–8
Abellanas MA, Zamarbide M, Basurco L, Luquin E, Garcia-Granero M, Clavero P, San Martin-Uriz P, Vilas A, Mengual E, Hervas-Stubbs S, Aymerich MS (2019) Midbrain microglia mediate a specific immunosuppressive response under inflammatory conditions. J Neuroinflammation 16:233
Kummer MP, Ising C, Kummer C, Sarlus H, Griep A, Vieira-Saecker A, Schwartz S, Halle A, Brückner M, Handler K, Schultze JL, Beyer M, Latz E, Heneka MT (2021) Microglial PD-1 stimulation by astrocytic PD-L1 suppresses neuroinflammation and Alzheimer’s disease pathology. EMBO J 40:e108662
He X, Lin S, Yang L, Tan P, Ma P, Qiu P, Zheng C, Zhang X, Kang W, Lin W (2021) Programmed death protein 1 is essential for maintaining the anti-inflammatory function of infiltrating regulatory T cells in a murine spinal cord injury model. J Neuroimmunol 354:577546
Cao Z, Harvey SS, Chiang T, Foltz AG, Lee AG, Cheng MY, Steinberg GK (2021) Unique subtype of microglia in degenerative thalamus after cortical stroke. Stroke 52:687–698
Bodhankar S, Chen YX, Vandenbark AA, Murphy SJ, Offner H (2013) PD-L1 enhances CNS inflammation and infarct volume following experimental stroke in mice in opposition to PD-1. J Neuroinflammation 10:111
Ren XF, Akiyoshi K, Vandenbark AA, Hurn PD (2011) Offner H (2011) Programmed death-1 pathway limits central nervous system inflammation and neurologic deficits in murine experimental stroke. Stroke 42:2578–2583
EL Shwetank F, Mockus TE, Ren HM, Toprak M, Lauver MD, Netherby-Winslow CS, Jin G, Cosby JM, Evavold BD, Lukacher AE (2019) PD-1 dynamically regulates inflammation and development of brain-resident memory CD8 T Cells During Persistent Viral Encephalitis. Front Immunol 10:783
Schachtele SJ, Hu SX, Sheng WS, Mutnal MB, Lokensgard GR (2014) Glial cells suppress postencephalitic CD8+ T lymphocytes through PD-L1. Glia 62:1582–1594
Dong L, Zheng YM, Luo XG, He ZY (2021) High inflammatory tendency induced by malignant stimulation through imbalance of CD28 and CTLA-4/PD-1 contributes to dopamine neuron injury. J Inflamm Res 14:2471–2482
Keikha M, Ghazvini K, Eslami M, Yousefi B, Casseb J, Yousefi M, Karbalaei M (2020) Molecular targeting of PD-1 signaling pathway as a novel therapeutic approach in HTLV-1 infection. Microb Pathog 144:104198
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (31600830, 31371374 and 31760279).
Funding
This research was supported by grants from the National Natural Science Foundation of China (31600830, 31371374 and 31760279). The funding bodies had no role in the design of the study or in the collection, analysis and interpretation of the data in this manuscript. National Natural Science Foundation of China,31600830,Liang-Wei Chen, 31371374, Liang-Wei Chen
Author information
Authors and Affiliations
Contributions
Ying-Ying Cheng and Bei-Yu Chen were involved in methodology, writing and preparing the original draft.
Gan-Lan Bian carried out formal analysis and validation.
Liang-Wei Chen and Yin-Xiu Ding contributed to conceptualization, and writing, reviewing and editing.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Animal experiments were conducted in accordance with the National Institute of Health guide for the care and use of Laboratory animals (NIH Publications, eighth edition revised in 2012) and under the approval of the Animal Experiment Administration Committee of the Fourth Military Medical University.
Consent for publication
All authors have read the manuscript and approved submission for publication.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Cheng, YY., Chen, BY., Bian, GL. et al. Programmed Death-1 Deficiency Aggravates Motor Dysfunction in MPTP Model of Parkinson's Disease by Inducing Microglial Activation and Neuroinflammation in Mice. Mol Neurobiol 59, 2642–2655 (2022). https://doi.org/10.1007/s12035-022-02758-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-022-02758-x