Abstract
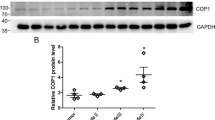
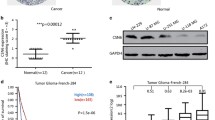
Constitutive photomorphogenic 1 (COP1, also known as RFWD2), a ring-finger-type E3 ubiquitin ligase, has been reported to play a pivotal role in the regulation of cell growth, apoptosis, and DNA repair. Accumulating evidence has suggested that COP1 plays a role in tumorigenesis by triggering the ubiquitination and degradation of its substrates, but the potential mechanism remains unclear. In this study, COP1 was used as a bait in a yeast two-hybrid experiment to screen COP1-interacting proteins in a human brain cDNA library, and the results indicated that protocadherin 9 (PCDH9) was a potential binding protein of COP1. The interaction between and colocalization of COP1 and PCDH9 was further confirmed by coimmunoprecipitation (co-IP) assay and immunofluorescent staining. Subsequently, we demonstrated that COP1 acted as an E3 ligase to promote the ubiquitination and degradation of PCDH9 through the proteasome pathway in glioma cells. Furthermore, we identified that the type of COP1 mediated PCDH9 ubiquitination was Lys48-linked polyubiquitination. Finally, we found that the COP1 protein level was inversely correlated with the PCDH9 protein level in human glioma tissues. Taken together, our results suggest that COP1 is an E3 ubiquitin ligase for PCDH9 and reveal an important mechanism for PCDH9 regulation in human glioma.




Similar content being viewed by others
Data Availability
All data generated or analyzed during this study are included in this published article.
Change history
17 May 2022
A Correction to this paper has been published: https://doi.org/10.1007/s12035-022-02865-9
References
Ostrom QT, Bauchet L, Davis FG, Deltour I, Fisher JL, Langer CE et al (2014) The epidemiology of glioma in adults: a “state of the science” review. Neuro Oncol 16(7):896–913
Furnari FB, Fenton T, Bachoo RM, Mukasa A, Stommel JM, Stegh A et al (2007) Malignant astrocytic glioma: genetics, biology, and paths to treatment. Genes Dev 21(21):2683–2710
Chen R, Smith-Cohn M, Cohen AL, Colman H (2017) Glioma subclassifications and their clinical significance. Neurotherapeutics 14(2):284–297
Norden AD, Wen PY (2006) Glioma therapy in adults. Neurologist 12(6):279–292
Meyer MA (2008) Malignant gliomas in adults. New Engl J Med 359(17):1850-
Mittal S, Pradhan S, Srivastava T (2015) Recent advances in targeted therapy for glioblastoma. Expert Rev Neurother 15(8):935–946
Clarke J, Butowski N, Chang S (2010) Recent advances in therapy for glioblastoma. Arch Neurol-Chicago 67(3):279–283
Bianchi E, Denti S, Catena R, Rossetti G, Polo S, Gasparian S et al (2003) Characterization of human constitutive photomorphogenesis protein 1, a ring finger ubiquitin ligase that interacts with jun transcription factors and modulates their transcriptional activity. J Biol Chem 278(22):19682–19690
Dornan D, Wertz I, Shimizu H, Arnott D, Frantz GD, Dowd P et al (2004) The ubiquitin ligase COP1 is a critical negative regulator of p53. Nature 429(6987):86–92
Deng XW, Caspar T, Quail PH (1991) cop1: a regulatory locus involved in light-controlled development and gene expression in Arabidopsis. Genes Dev 5(7):1172–1182
Ma L, Gao Y, Qu L, Chen Z, Li J, Zhao H et al (2002) Genomic evidence for COP1 as a repressor of light-regulated gene expression and development in arabidopsis. Plant Cell 14(10):2383–2398
Sanchez-Barcelo EJ, Mediavilla MD, Vriend J, Reiter RJ (2016) Constitutive photomorphogenesis protein 1 (COP1) and COP9 signalosome, evolutionarily conserved photomorphogenic proteins as possible targets of melatonin. J Pineal Res 61(1):41–51
Song Y, Liu Y, Pan S, Xie S, Wang Z-w, Zhu X (2020) Role of the COP1 protein in cancer development and therapy. Seminars in Cancer Biology 67(2):43–52
Ka WH, Cho SK, Chun BN, Byun SY, Ahn JC (2018) The ubiquitin ligase COP1 regulates cell cycle and apoptosis by affecting p53 function in human breast cancer cell lines. Breast Cancer-Tokyo 25(5):529–538
Marine JC (2012) Spotlight on the role of COP1 in tumorigenesis. Nat Rev Cancer 12(7):455–464
Kato S, Ding J, Pisck E, Jhala US, Du K (2008) COP1 Functions as a FoxO1 ubiquitin E3 ligase to regulate foxo1-mediated gene expression. J Biol Chem 283(51):35464–35473
Ko E-J, Oh YL, Kim HY, Eo WK, Kim H, Kim KH et al (2019) Correlation of constitutive photomorphogenic 1 (COP1) and p27 tumor suppressor protein expression in ovarian cancer. Genes & Genomics 41(8):879–884
Li DQ, Ohshiro K, Reddy SDN, Pakala SB, Lee MH, Zhang YP et al (2009) E3 ubiquitin ligase COP1 regulates the stability and functions of MTA1. P Natl Acad Sci USA 106(41):17493–17498
Dallavalle C, Albino D, Civenni G, Merulla J, Ostano P, Mello-Grand M et al (2016) MicroRNA-424 impairs ubiquitination to activate STAT3 and promote prostate tumor progression. J Clin Invest 126(12):4585–4602
Xu SS, Tong MH, Huang JQ, Zhang Y, Qiao YX, Weng WH et al (2014) TRIB2 inhibits Wnt/beta-Catenin/TCF4 signaling through its associated ubiquitin E3 ligases, beta-TrCP, COP1 and Smurf1, in liver cancer cells. Febs Lett 588(23):4334–4341
Dornan D, Bheddah S, Newton K, Ince W, Frantz GD, Dowd P et al (2004) COP1, the negative regulator of p53, is overexpressed in breast and ovarian adenocarcinomas. Cancer Res 64(20):7226–7230
Vitari AC, Leong KG, Newton K, Yee C, O’Rourke K, Liu JF et al (2011) COP1 is a tumour suppressor that causes degradation of ETS transcription factors. Nature 474(7351):403-+
Migliorini D, Bogaerts S, Defever D, Vyas R, Denecker G, Radaelli E et al (2011) Cop1 constitutively regulates c-Jun protein stability and functions as a tumor suppressor in mice. J Clin Invest 121(4):1329–1343
Su CH, Zhao RY, Zhang FM, Qu CJ, Chen B, Feng YH et al (2011) 14-3-3 sigma exerts tumor-suppressor activity mediated by regulation of COP1 stability. Cancer Res 71(3):884–894
Lee YH, Andersen JB, Song HT, Judge AD, Seo D, Ishikawa T et al (2010) Definition of ubiquitination modulator COP1 as a novel therapeutic target in human hepatocellular carcinoma. Cancer Res 70(21):8264–8269
Li YF, Wang DD, Zhao BW, Wang W, Huang CY, Chen YM et al (2012) High level of COP1 expression is associated with poor prognosis in primary gastric cancer. Int J Biol Sci 8(8):1168–1177
Zou SS, Zhu YF, Wang B, Qian FY, Zhang X, Wang L et al (2017) The ubiquitin ligase COP1 promotes glioma cell proliferation by preferentially downregulating tumor suppressor p53. Mol Neurobiol 54(7):5008–5016
Kim SY, Yasuda S, Tanaka H, Yamagata K, Kim H (2011) Non-clustered protocadherin. Cell Adhes Migr 5(2):97–105
Frank M, Kemler R (2002) Protocadherins. Curr Opin Cell Biol 14(5):557–562
Redies C, Vanhalst K, Roy F (2005) delta-protocadherins: unique structures and functions. Cell Mol Life Sci 62(23):2840–2852
Ashaie MA, Chowdhury EH (2016) Cadherins: the superfamily critically involved in breast cancer. Curr Pharm Design 22(5):616–638
van Roy F (2014) Beyond E-cadherin: roles of other cadherin superfamily members in cancer. Nat Rev Cancer 14(2):121–134
Sui XB, Wang D, Geng SM, Zhou GL, He C, Hu XT (2012) Methylated promoters of genes encoding protocadherins as a new cancer biomarker family. Mol Biol Rep 39(2):1105–1111
El Hajj N, Dittrich M, Haaf T (2017) Epigenetic dysregulation of protocadherins in human disease. Semin Cell Dev Biol 69:172–182
Seim I, Jeffery PL, Thomas PB, Nelson CC, Chopin LK (2017) Whole-genome sequence of the metastatic PC3 and LNCaP human prostate cancer cell lines. G3-Genes Genom Genet 7(6):1731–41
Chen Y, Xiang HG, Zhang YF, Wang JJ, Yu GZ (2015) Loss of PCDH9 is associated with the differentiation of tumor cells and metastasis and predicts poor survival in gastric cancer. Clin Exp Metastas 32(5):417–428
Zhu PF, Lv J, Yang ZW, Guo LM, Zhang L, Li M et al (2014) Protocadherin 9 inhibits epithelial-mesenchymal transition and cell migration through activating GSK-3 beta in hepatocellular carcinoma. Biochem Bioph Res Co 452(3):567–574
Wang CL, Yu GZ, Liu JC, Wang JB, Zhang YM, Zhang X et al (2012) Downregulation of PCDH9 predicts prognosis for patients with glioma. J Clin Neurosci 19(4):541–545
Lv J, Zhu PF, Zhang XL, Zhang L, Chen XM, Lu FM et al (2017) PCDH9 acts as a tumor suppressor inducing tumor cell arrest at G(0)/G(1) phase and is frequently methylated in hepatocellular carcinoma. Mol Med Rep 16(4):4475–4482
Izycka N, Sterzynska K, Januchowski R, Nowak-Markwitz E (2019) Semaphorin 3A (SEMA3A), protocadherin 9 (PCDH9), and S100 calcium binding protein A3 (S100A3) as potential biomarkers of carcinogenesis and chemoresistance of different neoplasms, including ovarian cancer - review of literature. Ginekol Pol 90(4):223–227
Wang CL, Chen Q, Li S, Li ST, Zhao ZY, Gao HL et al (2017) Dual inhibition of PCDH9 expression by miR-215-5p up-regulation in gliomas. Oncotarget 8(6):10287–10297
Shi C, Yang YJ, Zhang L, Yu JP, Qin SS, Xu HG et al (2019) MiR-200a-3p promoted the malignant behaviors of ovarian cancer cells through regulating PCDH9. Oncotargets Ther 12:8329–8338
Tayrac Md, Etcheverry A, Aubry M, Saïkali S, Hamlat A, Quillien V et al (2009) Integrative genome-wide analysis reveals a robust genomic glioblastoma signature associated with copy number driving changes in gene expression. Gene Chromosom Cancer 48(1):55–68
Fu C, Gong Y, Shi X, Shi H, Wan Y, Wu Q, et al (2016) Expression and regulation of COP1 in chronic lymphocytic leukemia cells for promotion of cell proliferation and tumorigenicity. 35(3):1493–500.
Li J, Wang L, Xiao R, Pan Q, Huang H, Kuang R (2016) High expression of constitutive photomorphogenic 1 (COP1) is associated with poor prognosis in bladder cancer. Tumor Biology 37(7):8917–8922
Choi HH, Phan L, Chou PC, Su CH, Yeung SCJ, Chen JS et al (2015) COP1 enhances ubiquitin-mediated degradation of p27(Kip1) to promote cancer cell growth. Oncotarget 6(23):19721–19734
Wong C-M, Jiang S, Yang Z, Li W, Li X, Wang Y, et al (2012) Re-evaluation of the carcinogenic significance of hepatitis B virus integration in Hepatocarcinogenesis. PLoS ONE. 7(9)
Acknowledgements
We thank Chunling Fu (Blood Diseases Institute, Xuzhou Medical University ) for helping with some experiments and critical comment on the manuscript.
Funding
This study was supported by the National Natural Science Foundation of China (81874081), the Young Science and Technology Innovation Team of Xuzhou Medical University (TD202006), the Foundation of Xuzhou Science and Technology Bureau (KC20139, KC20128, KC20091), the Jiangsu Provincial Qing Lan Project and Jiangsu Provincial Medical Youth Talent (QNRC2016784), the Foundation of Jiangsu Provincial Health Department (M2020082), the National Demonstration Center for Experimental Basic Medical Science Education (Xuzhou Medical University), the National Innovation and Entrepreneurship Training Program for College Students (201910313026 and 201910313019Z), and the Natural Science Foundation of Jiangsu Province of China (BK20181149).
Author information
Authors and Affiliations
Contributions
Conceptualization, Hengliang Shi. Funding acquisition, Hengliang Shi, Bin Zhang. Investigation, Kunlin Zhou and Lei Wang. Methodology, Zhiyuan Sun, Yufu Zhu, Yuelin Liu, and Zhiyi Liu. Supervision, Hengliang Shi. Writing—original draft, Kunlin Zhou. Writing—review and editing, Hengliang Shi. All authors made substantial contributions to this study.
Corresponding authors
Ethics declarations
Ethics Approval
The experimental protocol for human glioma tissues were obtained from the Affiliated Hospital of Xuzhou Medical University. This study was approved by the Ethics Committee of the Affiliated Hospital of Xuzhou Medical University.
Consent to Participate
Glioma patients signed an internal regulatory document, stating that residual samples used for diagnostic procedures can be used for retrospective academic studies, without any additional informed consent.
Consent for Publication
Not applicable.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhou, K., Wang, L., Sun, Z. et al. COP1 Acts as a Ubiquitin Ligase for PCDH9 Ubiquitination and Degradation in Human Glioma. Mol Neurobiol 59, 2378–2388 (2022). https://doi.org/10.1007/s12035-021-02634-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-021-02634-0