Abstract
Stress contributes to major depressive disorder (MDD) and chronic pain, which affect a significant portion of the global population, but researchers have not clearly determined how these conditions are initiated or amplified by stress. The chronic social defeat stress (CSDS) model is a mouse model of psychosocial stress that exhibits depressive-like behavior and chronic pain. We hypothesized that metabotropic glutamate receptor 5 (mGluR5) expressed in the nucleus accumbens (NAc) normalizes the depressive-like behaviors and pain following CSDS. Here, we show that CSDS induced both pain and social avoidance and that the level of mGluR5 decreased in susceptible mice. Overexpression of mGluR5 in the NAc shell and core prevented the development of depressive-like behaviors and pain in susceptible mice, respectively. Conversely, depression-like behaviors and pain were exacerbated in mice with mGluR5 knockdown in the NAc shell and core, respectively, compared to control mice subjected to 3 days of social defeat stress. Furthermore, (RS)-2-chloro-5-hydroxyphenylglycine (CHPG), an mGluR5 agonist, reversed the reduction in the level of the endocannabinoid (eCB) 2-arachidonoylglycerol (2-AG) in the NAc of susceptible mice, an effect that was blocked by 3-((2-methyl-1, 3-thiazol-4-yl) ethynyl) pyridine hydrochloride (MTEP), an mGluR5 antagonist. In addition, the injection of CHPG into the NAc shell and core normalized depressive-like behaviors and pain, respectively, and these effects were inhibited by AM251, a cannabinoid type 1 receptor (CB1R) antagonist. Based on these results, mGluR5-mediated eCB production in the NAc relieves stress-induced depressive-like behaviors and pain.
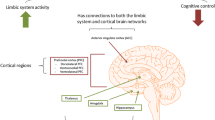
Graphical abstract










Similar content being viewed by others
Data Availability
All the data supporting the findings of this study are available within the article and its Supplementary Information files and from the corresponding author upon reasonable request.
Abbreviations
- MDD:
-
Major depressive disorder
- CSDS:
-
Chronic social defeat stress
- NAc:
-
Nucleus accumbens
- eCB:
-
Endocannabinoid
- 2-AG:
-
2-Arachidonoylglycerol
- CHPG:
-
(RS)-2-chloro-5-hydroxyphenylglycine
- MTEP:
-
3-((2-Methyl-1,3-thiazol-4-yl)ethynyl) pyridine hydrochloride
- CB1R :
-
Cannabinoid type 1 receptor
- mGluR5:
-
Metabotropic glutamate receptor 5
- mPFC:
-
Medial prefrontal cortex
- AEA :
-
Anandamide
- LTD:
-
Long-term depression
- SPT:
-
Sucrose preference test
- TST:
-
Tail suspension test
References
Pagliusi M Jr, Bonet IJM, Brandao AF, Magalhaes SF, Tambeli CH, Parada CA, Sartori CR (2020) Therapeutic and preventive effect of voluntary running wheel Exercise on social defeat stress (SDS)-induced depressive-like behavior and chronic pain in mice. Neuroscience 428:165–177
Aaron RV, Fisher EA, de la Vega R, Lumley MA, Palermo TM (2019) Alexithymia in individuals with chronic pain and its relation to pain intensity, physical interference, depression, and anxiety: a systematic review and meta-analysis. Pain 160(5):994–1006
de Waal MW, Hegeman JM, Gussekloo J, Verhaak PF, van der Mast RC, Comijs HC (2016) The effect of pain on presence and severity of depressive disorders in older persons: the role of perceived control as mediator. J Affect Disord 197:239–244
Kudryavtseva NN, Bakshtanovskaya IV, Koryakina LA (1991) Social model of depression in mice of C57BL/6J strain. Pharmacol Biochem Behav 38(2):315–320
Krishnan V, Han MH, Graham DL, Berton O, Renthal W, Russo SJ, Laplant Q, Graham A et al (2007) Molecular adaptations underlying susceptibility and resistance to social defeat in brain reward regions. Cell 131(2):391–404
Lim BK, Huang KW, Grueter BA, Rothwell PE, Malenka RC (2012) Anhedonia requires MC4R-mediated synaptic adaptations in nucleus accumbens. Nature 487(7406):183–189
Wagner KV, Hartmann J, Labermaier C, Hausl AS, Zhao G, Harbich D, Schmid B, Wang XD et al (2015) Homer1/mGluR5 activity moderates vulnerability to chronic social stress. Neuropsychopharmacology 40(5):1222–1233
Strain JJ (2018) The psychobiology of stress, depression, adjustment disorders and resilience. World J Biol Psychiatry 19(sup1):S14–S20
Pagliusi MOF Jr, Bonet IJM, Dias EV, Vieira AS, Tambeli CH, Parada CA, Sartori CR (2018) Social defeat stress induces hyperalgesia and increases truncated BDNF isoforms in the nucleus accumbens regardless of the depressive-like behavior induction in mice. Eur J Neurosci 48:1635–1646
Robinson MJ, Edwards SE, Iyengar S, Bymaster F, Clark M, Katon W (2009) Depression and pain. Front Biosci (Landmark Ed) 14:5031–5051
Goldenberg DL (2010) Pain/depression dyad: a key to a better understanding and treatment of functional somatic syndromes. Am J Med 123(8):675–682
Massaly N, Copits BA, Wilson-Poe AR, Hipolito L, Markovic T, Yoon HJ, Liu S, Walicki MC et al (2019) Pain-induced negative affect is mediated via recruitment of the nucleus accumbens kappa opioid system. Neuron 102(3):564–573 e6
Heshmati M, Aleyasin H, Menard C, Christoffel DJ, Flanigan ME, Pfau ML, Hodes GE, Lepack AE et al (2018) Cell-type-specific role for nucleus accumbens neuroligin-2 in depression and stress susceptibility. Proc Natl Acad Sci U S A 115(5):1111–1116
Niswender CM, Conn PJ (2010) Metabotropic glutamate receptors: physiology, pharmacology, and disease. Annu Rev Pharmacol Toxicol 50:295–322
Kim CH, Lee J, Lee JY, Roche KW (2008) Metabotropic glutamate receptors: phosphorylation and receptor signaling. J Neurosci Res 86(1):1–10
Guo B, Wang J, Yao H, Ren K, Chen J, Yang J, Cai G, Liu H et al (2018) Chronic inflammatory pain impairs mGluR5-mediated depolarization-induced suppression of excitation in the anterior cingulate cortex. Cereb Cortex 28(6):2118–2130
Smith ACW, Scofield MD, Heinsbroek JA, Gipson CD, Neuhofer D, Roberts-Wolfe DJ, Spencer S, Garcia-Keller C et al (2017) Accumbens nNOS interneurons regulate cocaine relapse. J Neurosci 37(4):742–756
Jiang X, Lin W, Cheng Y, Wang D (2020) mGluR5 facilitates long-term synaptic depression in a stress-induced depressive mouse model. Can J Psychiatr 65(5):347–355
Shin S, Kwon O, Kang JI, Kwon S, Oh S, Choi J, Kim CH, Kim DG (2015) mGluR5 in the nucleus accumbens is critical for promoting resilience to chronic stress. Nat Neurosci 18(7):1017–1024
Chung G, Kim SJ, Kim SK (2018) Metabotropic glutamate receptor 5 in the medial prefrontal cortex as a molecular determinant of pain and ensuing depression. Front Mol Neurosci 11:376
Chung G, Kim CY, Yun YC, Yoon SH, Kim MH, Kim YK, Kim SJ (2017) Upregulation of prefrontal metabotropic glutamate receptor 5 mediates neuropathic pain and negative mood symptoms after spinal nerve injury in rats. Sci Rep 7(1):9743
Bisogno T, Berrendero F, Ambrosino G, Cebeira M, Ramos JA, Fernandez-Ruiz JJ, Di Marzo V (1999) Brain regional distribution of endocannabinoids: implications for their biosynthesis and biological function. Biochem Biophys Res Commun 256(2):377–380
Fitzgibbon M, Kerr DM, Henry RJ, Finn DP, Roche M (2019) Endocannabinoid modulation of inflammatory hyperalgesia in the IFN-alpha mouse model of depression. Brain Behav Immun 82:372–381
Wilson RI, Nicoll RA (2001) Endogenous cannabinoids mediate retrograde signalling at hippocampal synapses. Nature 410(6828):588–592
Robbe D, Kopf M, Remaury A, Bockaert J, Manzoni OJ (2002) Endogenous cannabinoids mediate long-term synaptic depression in the nucleus accumbens. Proc Natl Acad Sci U S A 99(12):8384–8388
Maccarrone M, Rossi S, Bari M, De Chiara V, Fezza F, Musella A, Gasperi V, Prosperetti C et al (2008) Anandamide inhibits metabolism and physiological actions of 2-arachidonoylglycerol in the striatum. Nat Neurosci 11(2):152–159
Maejima T, Oka S, Hashimotodani Y, Ohno-Shosaku T, Aiba A, Wu D, Waku K, Sugiura T et al (2005) Synaptically driven endocannabinoid release requires Ca2+-assisted metabotropic glutamate receptor subtype 1 to phospholipase Cbeta4 signaling cascade in the cerebellum. J Neurosci 25(29):6826–6835
Zhu PJ, Lovinger DM (2005) Retrograde endocannabinoid signaling in a postsynaptic neuron/synaptic bouton preparation from basolateral amygdala. J Neurosci 25(26):6199–6207
Orlando LR, Dunah AW, Standaert DG, Young AB (2002) Tyrosine phosphorylation of the metabotropic glutamate receptor mGluR5 in striatal neurons. Neuropharmacology 43(2):161–173
Golden SA, Covington HE 3rd, Berton O, Russo SJ (2011) A standardized protocol for repeated social defeat stress in mice. Nat Protoc 6(8):1183–1191
Guo M, Lu Y, Garza JC, Li Y, Chua SC, Zhang W, Lu B, Lu XY (2012) Forebrain glutamatergic neurons mediate leptin action on depression-like behaviors and synaptic depression. Transl Psychiatry 2:e83
Qin Z, Zhou X, Pandey NR, Vecchiarelli HA, Stewart CA, Zhang X, Lagace DC, Brunel JM et al (2015) Chronic stress induces anxiety via an amygdalar intracellular cascade that impairs endocannabinoid signaling. Neuron 85(6):1319–1331
Li MX, Zheng HL, Luo Y, He JG, Wang W, Han J, Zhang L, Wang X et al (2018) Gene deficiency and pharmacological inhibition of caspase-1 confers resilience to chronic social defeat stress via regulating the stability of surface AMPARs. Mol Psychiatry 23(3):556–568
Bosch-Bouju C, Larrieu T, Linders L, Manzoni OJ, Laye S (2016) Endocannabinoid-mediated plasticity in nucleus accumbens controls vulnerability to anxiety after social defeat stress. Cell Rep 16(5):1237–1242
Devane WA, Hanus L, Breuer A, Pertwee RG, Stevenson LA, Griffin G, Gibson D, Mandelbaum A et al (1992) Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science 258(5090):1946–1949
Herkenham M, Lynn AB, Little MD, Johnson MR, Melvin LS, de Costa BR, Rice KC (1990) Cannabinoid receptor localization in brain. Proc Natl Acad Sci U S A 87(5):1932–1936
Matsuda LA, Lolait SJ, Brownstein MJ, Young AC, Bonner TI (1990) Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature 346(6284):561–564
Sugiura T, Kondo S, Sukagawa A, Nakane S, Shinoda A, Itoh K, Yamashita A, Waku K (1995) 2-Arachidonoylglycerol: a possible endogenous cannabinoid receptor ligand in brain. Biochem Biophys Res Commun 215(1):89–97
Bradley JJ (1963) Severe localized pain associated with the depressive syndrome. Br J Psychiatry 109:741–745
Gerrits MM, Vogelzangs N, van Oppen P, van Marwijk HW, van der Horst H, Penninx BW (2012) Impact of pain on the course of depressive and anxiety disorders. Pain 153(2):429–436
Atwal N, Winters BL, Vaughan CW (2020) Endogenous cannabinoid modulation of restraint stress-induced analgesia in thermal nociception. J Neurochem 152(1):92–102
Marsden WN (2013) Synaptic plasticity in depression: molecular, cellular and functional correlates. Prog Neuro-Psychopharmacol Biol Psychiatry 43:168–184
Duman RS, Aghajanian GK, Sanacora G, Krystal JH (2016) Synaptic plasticity and depression: new insights from stress and rapid-acting antidepressants. Nat Med 22(3):238–249
Bannerman DM, Sprengel R, Sanderson DJ, McHugh SB, Rawlins JN, Monyer H, Seeburg PH (2014) Hippocampal synaptic plasticity, spatial memory and anxiety. Nat Rev Neurosci 15(3):181–192
Kim SK, Hayashi H, Ishikawa T, Shibata K, Shigetomi E, Shinozaki Y, Inada H, Roh SE et al (2016) Cortical astrocytes rewire somatosensory cortical circuits for peripheral neuropathic pain. J Clin Invest 126(5):1983–1997
Cordeiro Matos S, Zhang Z, Seguela P (2015) Peripheral neuropathy induces HCN channel dysfunction in pyramidal neurons of the medial prefrontal cortex. J Neurosci 35(38):13244–13256
Kolber BJ (2015) mGluRs head to toe in pain. Prog Mol Biol Transl Sci 131:281–324
Martinez E, Lin HH, Zhou H, Dale J, Liu K, Wang J (2017) Corticostriatal regulation of acute pain. Front Cell Neurosci 11:146
de Laat B, Leurquin-Sterk G, Celen S, Bormans G, Koole M, Van Laere K, Casteels C (2015) Preclinical evaluation and quantification of 18F-FPEB as a radioligand for PET imaging of the metabotropic glutamate receptor 5. J Nucl Med 56(12):1954–1959
Guindon J, Hohmann AG (2009) The endocannabinoid system and pain. CNS Neurol Disord Drug Targets 8(6):403–421
Di Marzo V (2011) Endocannabinoid signaling in the brain: biosynthetic mechanisms in the limelight. Nat Neurosci 14(1):9–15
Kiritoshi T, Ji G, Neugebauer V (2016) Rescue of impaired mGluR5-driven endocannabinoid signaling restores prefrontal cortical output to inhibit pain in arthritic rats. J Neurosci 36(3):837–850
Mineur YS, Belzung C, Crusio WE (2006) Effects of unpredictable chronic mild stress on anxiety and depression-like behavior in mice. Behav Brain Res 175(1):43–50
Wang W, Sun D, Pan B, Roberts CJ, Sun X, Hillard CJ, Liu QS (2010) Deficiency in endocannabinoid signaling in the nucleus accumbens induced by chronic unpredictable stress. Neuropsychopharmacology 35(11):2249–2261
Lomazzo E, Bindila L, Remmers F, Lerner R, Schwitter C, Hoheisel U, Lutz B (2015) Therapeutic potential of inhibitors of endocannabinoid degradation for the treatment of stress-related hyperalgesia in an animal model of chronic pain. Neuropsychopharmacology 40(2):488–501
Hill MN, Miller GE, Carrier EJ, Gorzalka BB, Hillard CJ (2009) Circulating endocannabinoids and N-acyl ethanolamines are differentially regulated in major depression and following exposure to social stress. Psychoneuroendocrinology 34(8):1257–1262
Funding
This work was supported by a grant from the Natural Science Foundation of Shanghai to T.X. (21ZR1448400), the Interdisciplinary Program of Shanghai Jiao Tong University to T.X. (grant no. YG2021ZD23), a grant from the Natural Science Foundation of China to C.S. (81901141), and grants-in-aid from Shanghai Municipal Commission of Science and Technology (18DZ2260200).
Author information
Authors and Affiliations
Contributions
X.X., K.W., X.M., W.W., data curation, investigation, and methodology; H.W., M.H., methodology and software; H.S. and T.Y., formal analysis; S.C., visualization and funding acquisition; J.H., A.W. and T.X., writing and supervision.
Corresponding authors
Ethics declarations
Ethics Approval
All experiments and procedures were conducted in accordance with the Guide for the Care and Use of Laboratory Animals (Eighth Edition) published by the National Research Council (USA) and were approved by the Institutional Animal Care and Use Committee of Sixth People’s Hospital Affiliated with Shanghai Jiao Tong University.
Consent to Participate
Not applicable
Consent for Publication
Not applicable
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
• Chronic social defeat stress-induced depressive-like behaviors and pain by decreasing mGluR5 levels in the nucleus accumbens.
• Overexpression or activation of mGluR5 in the nucleus accumbens prevented the development of depressive-like behaviors and pain following stress.
• The enhancement of endocannabinoid signaling in the NAc by targeted pharmacological activation of mGluR5 in the NAc alleviates depressive-like behaviors and relieves pain in mice exposed to stress.
Supplementary Information
ESM 1
(PNG 5663 kb)
Rights and permissions
About this article
Cite this article
Xu, X., Wu, K., Ma, X. et al. mGluR5-Mediated eCB Signaling in the Nucleus Accumbens Controls Vulnerability to Depressive-Like Behaviors and Pain After Chronic Social Defeat Stress. Mol Neurobiol 58, 4944–4958 (2021). https://doi.org/10.1007/s12035-021-02469-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-021-02469-9