Abstract
Psychedelics are compounds acting by serotonin 5-hydroxytryptamine (5-HT)2A receptor activation and induce several behavioral responses. They are of special interest because of their positive effects on neuropsychiatric disorders (depression and posttraumatic stress disorder). However, several findings revealed that some psychedelic actions are similar to symptoms observed in schizophrenia (psychosis, sensorimotor gating impairments, attention, and working memory deficits) which might limit their clinical applications. Psychedelics activate some neurotransmitters, i.e., serotonergic, and glutamatergic, that are also impaired in schizophrenia. Therefore, the neurobiological background of psychedelics and schizophrenia is partially similar. Another important aspect to discuss is the perspective of using psychedelics in schizophrenia therapy. Postmortem studies showed a loss of synapses in schizophrenia, and the positive effects of psychedelics on neuroplasticity (synaptogenesis, neurogenesis, and neuritogenesis) might be essential in the context of schizophrenia therapy. However, because of psychedelics' psychotic action, the recommended doses of psychedelics in schizophrenia treatment are not established, and subpsychedelic dosing or microdosing are considered. Exploratory studies are needed to determine the tolerability of treatment and appropriate dosing regimen. Another therapeutic option is using non-hallucinogenic psychedelic analogs that also induce neuroplastic outcomes but do not have psychotogenic effects. Further preclinical and clinical studies are needed to recognize the potential effectiveness of 5-HT2A agonists in schizophrenia therapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Psychedelics (mind-manifesting) are psychoactive substances that induce alterations in mood, thought processes, perceptions, and experiences rarely experienced except in dreams, contemplative and exaltation, and acute psychosis (known as psychedelic experiences or psychedelic trips). They are also called psychotomimetic (psychosis-mimicking) or psycholytic (psyche-loosing) [1]. Most psychedelics derive from plants or semisynthetic and can be classified by chemical structure into indolamines and phenethylamines. The indolamine group includes lysergic acid diethylamide (LSD), semisynthetic ergosterol that naturally occurs as ergot alkaloid lysergic acid in the rye parasite; psilocybin, a compound found in magic mushrooms, N,N-dimethyltryptamine (DMT), the active ingredient in ayahuasca; 5-methoxy-N, N-dimethyltryptamine (5-MeO-DMT), the active ingredient in morning glories. The phenethylamine group includes mescaline, the active ingredient in the peyote and Sant Pedro cacti, and 2,5-dimethoxy-4-iodoamphetamine (DOI) [2].
The subjective effects of psychedelics have been assessed using the Hallucinogen Rating Scale (HRS) and Abnormal Mental States (APZ) questionnaire to measure altered states of consciousness (ASC) [3]. HRS elements were placed into six conceptually coherent clusters: somaesthesia, affect, perception, cognition, volition, and intensity. ASCs are defined by three primary dimensions: oceanic boundlessness (OSE, OBN, and OB), dread of ego dissolution (AIA, DED, and AED), and visionary restructuralization (VUS, VRS, and VR). The first dimension (oceanic boundlessness) measures derealization and depersonalization phenomena associated with positive basic mood. The second dimension (dread of ego dissolution) measures thought disorder, ego disintegration, and loss of autonomy and self-control associated with arousal, anxiety, and paranoid feelings. The third dimension (visionary restructuralization) is related to auditory and visual illusions, hallucinations, synaesthesias, and alterations in the meaning of various percepts [4]. ASC scale was revised to OAV scales, and later to the five-dimensional altered states consciousness (5AD-ASC) [3].
Psychedelics are classic serotonergic hallucinogens acting as an agonist or a partial agonist of the serotonin 5-hydroxytryptamine (5-HT)2A receptor. Pharmacological studies showed that psychedelic effects in humans depend on the activation of 5HT2A receptors, but not dopamine D2 receptors. Pretreatment with ketanserin, an antagonist of 5-HT2A/2C receptors, effectively blocked the effects of psilocybin on all three dimensions of ASC. Likewise, pretreatment with an atypical antipsychotic, a mixed 5-HT2A/D2 antagonist, risperidone attenuated APZ scores in the psilocybin study, but no effect was observed after administration of a typical antipsychotic, haloperidol, a D2 receptor antagonist [5]. Neuroimaging studies in humans also showed significant psilocybin 5-HT2A receptor occupancy in the brain that correlated with the psychedelic experience [6].
5-HT2A receptors
5-HT2A receptor is mainly expressed in the frontal cortex of humans and rodents, and it is also detectable at relatively lower densities in other brain regions, i.e., hippocampus, thalamus, and basal ganglia. In the cortex, 5-HT2A receptors are predominantly found on the dendrites of excitatory glutamatergic pyramidal neurons [7,8,9].
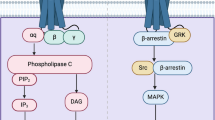
There are known several 5-HT2A receptor-coupled signaling pathways. The best-characterized signaling pathway is Gq/11-mediated activation of a cytoplasmic protein phospholipase C (PLC) that cleaves phosphatidylinositol 4,5-biphosphate (PIP2), a phospholipid in the plasma membrane, followed by a generation of diacylglycerol (DAG) and inositol triphosphate (IP3). IP3 evokes the release of CA2+ from the endoplasmic reticulum, and the stimulation of protein kinase C (PKC) [10,11,12,13]. The 5-HT2A receptor can also bind to other G proteins and downstream effector pathways. The 5-HT2A receptor agonists stimulate phospholipase A2 (PLA2), and the release of arachidonic acid (AA). The activation of PLA2 is not dependent on Gq/11 stimulation but correlates with Gi/o pathway and Src, and G12/13 activation of Rho [14,15,16]. Activation of the 5-HT2A receptor also induces Gi/o-dependent Gβγ-associates stimulation of extracellular signal-regulated kinase, p44/p42 (ERK1/2) [17]. There are several additional pathways linked to 5-HT2A receptor stimulation, i.e., pERK through β-arrestin [18] or phospholipase D (PLD) by the small G-protein ADP-ribosylation factor (SRF) activation [19].
Several findings indicate that hallucinogenic responses caused by 5-HT2A receptor agonists depend on the activation of specific signaling pathways. In vitro study demonstrated that hallucinogenic and non-hallucinogenic 5-HT2A receptor agonists stimulate PLC signaling pathways, while hallucinogen-dependent reactions also include pertussis toxin (PTX)-sensitive heterotrimeric Gi/o proteins [20]. The results from in vivo and in vitro studies indicate that hallucinogens selectively activate Gi/o-dependent signaling, whereas non-hallucinogenic 5-HT2A agonists do not stimulate Gi/o [21].
Biased agonism of the 5-HT2A receptor was also observed at the transcription level. Both hallucinogenic and non-hallucinogenic 5-HT2A agonists induced c-Fos and IκBα transcripts. However, transcripts Egr-1 and Egr-2 were activated only by hallucinogens (DOI, DOM, DOB, mescaline, LSD, psilocin), but not by non-hallucinogenic 5-HT2A agonists (R-lisuride, S-lisuride, ergotamine) [20, 22]. Hallucinogen-characteristic transcriptome fingerprints were dependent on the modulation of both Gq/11 and Gi/o. Recent findings indicate that hallucinogenic-like behavioral responses to 5-HT2A agonists (head twitch in animals) seem to be related to the activation of Gq and Gs pathways. Hallucinogen agonists (DOM, 25C-NBOH) activate both Gq and Gs proteins and produce a head twitch response. In contrast, non-hallucinogenic agonists (lisuride and TBG) only activate Gq signaling and do not induce hallucinogenic-like behavior [23].
The above observation suggests that in the case of the 5-HT2A receptor, a biased agonism related to the diverse signal transduction pathways stimulated by 5-HT2A receptor agonists is detected. Thus, the distinct behavioral and molecular responses produced by hallucinogenic and non-hallucinogenic 5-HT2A receptor agonists acting by the same population of cortical pyramidal 5-HT2A receptors might be explained by that phenomenon.
Schizophrenia
Schizophrenia is a severe, chronic disorder with symptoms divided into three groups: positive symptoms (delusions, hallucinations, inexplicable behavioral changes, and thought disorders), negative symptoms (avolition, anhedonia, asociality, blunted affect, and alogia), and cognitive deficits (poor learning, attention and working memory impairments, and retention of verbal information) [24,25,26].
Several findings indicate that schizophrenia is a neurodevelopmental disorder with first symptoms observed in late adolescence or young adulthood. The age of schizophrenia appearance depends on sex. In the case of men, the first signs are observed around the late teenagers to early 20s, in women between 20s and early 30s [27]. The epidemiological studies suggest the beginning of negative symptoms approximately five years before the initial psychotic episode, and the positive symptoms start close to the first hospitalization [28, 29].
The etiology of schizophrenia is still under investigation. However, epidemiologic and genetic evidence suggests that disease development results from the interaction of genetic background with environmental conditions. The above factors affect brain maturation during early life and adolescence [30]. Moreover, impaired epigenetic mechanisms, interacting with environmental risk factors and controlling gene expression, are also considered in the etiology of schizophrenia [31].
The pathophysiology of schizophrenia is still being explored. However, several neuroanatomical and neurotransmitter abnormalities are detected in schizophrenia. Neuroanatomical studies showed no changes in the total number of neurons in schizophrenia but an increase in neuronal density and a reduction in the neuropil were detected. Shorter dendritic length, lower dendritic spines on pyramidal neurons, and a decrease in the level of synaptophysin, a marker of axon terminals, and lower synaptic vesicle density are also noticed [32,33,34,35,36]. Some evidence shows 60% synapse loss in schizophrenia [37]. The schizophrenia hypothesis suggests that overpruning in synapses, mainly glutamatergic inputs onto cortical interneurons, disrupts excitation/inhibition (E/I) balance resulting in negative and cognitive symptoms development [38].
The above hypothesis is supported by neuroanatomical changes implying dysfunction in neurotransmitters, mainly excitatory ones. Alterations in the glutamate signaling might be related to the dysfunction of glutamatergic receptors i.e., the N-methyl-d-aspartate (NMDA) receptor, α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic (AMPA) receptor, or metabotropic (mGlu) receptor. Genetic studies specified genes coding glutamate receptor subunits, GRIN2A for NMDA receptor, GRIA1, GRIA3 for AMPA receptor, or GRM3 for mGlu3 receptor as schizophrenia risk genes [39]. Moreover, the hypofunction of the NMDA receptor is postulated based on the effects of non-competitive NMDA receptor antagonists, ketamine, and phencyclidine (PCP). They were originally developed as dissociative anesthetics, but they are known to induce schizophrenia-like abnormalities in healthy humans. The symptoms include not only positive ones (psychosis, thought disorder) but also negative and cognitive deficits [40]. Antipsychotic effects of drugs acting selectively through glutamatergic signaling have been explored. Classical agonists of the NMDA receptor cause excitotoxicity and neuron damage, and they are not considered in schizophrenia treatment However, stimulation of NMDA receptor indirectly by glycinergic (serine, cycloserine) acting as NMDA coagonists and glycine transport (GlyT1) inhibitors (bitopertin) have been studied. In clinical trials, they demonstrate some effects on negative symptoms of schizophrenia [41]. Positive allosteric modulators of AMPA or mGlu receptors and mGlu agonists are also promising targets for new antipsychotic drugs. However, they are still under investigation and need evaluation in clinical trials [42, 43].
Another hypothesis proposes that disinhibition of excitatory cortical projection induces dysregulation of mesostriatal dopamine neurons and psychotic symptoms [38]. Positive symptoms result from hyperactivity in dopaminergic transmission in limbic pathways, while negative symptoms are thought to arise from hypodopaminergic functioning in the frontal structures. These interpretations are supported by the fact that all antipsychotics have an affinity to the D2 receptor and most of them are antagonists to this receptor [44]. Moreover, gene-associated studies showed a possible relationship between the risk of schizophrenia and some variants in genes related to dopamine signaling, i.e., catechol-O-methyltransferase (COMT), monoamine oxidase (MAO), dopamine transporter (SLC6A3), and dystrobrevin-binding protein 1 (DTNBP1) [39].
The role of serotonin, the especially 5-HT2A receptor in schizophrenia pathogenesis is also considered. This hypothesis is based on the fact that psychedelics causing psychosis are agonists of the 5-HT2A receptor. On the other hand, 5-HT2A antagonists might have antipsychotic properties, mainly in negative symptoms [44]. Alternative 5-HT targets are also investigated in the context of schizophrenia treatment, i.e., 5-HT1A agonists, 5-HT reuptake inhibitors, 5-HT2C antagonists and agonists, 5-HT3 antagonists, 5-HT6 antagonists, 5-HT7 antagonists. However, there are still no drugs with selective serotonin pharmacological profile used in schizophrenia therapy [41].
Postmortem studies examining the level of 5-HT2A protein showed inconsistent results. The [3H] ketanserin binding study revealed a decrease in the density of 5-HT2A receptors in cortical areas of schizophrenia patients [45]. However, another [3H] ketanserin binding examination reported an increase in the number of [3H] ketanserin binding sites to the 5-HT2A receptor in the postmortem prefrontal cortex of antipsychotic-free but not in antipsychotic-treated schizophrenic subjects [46]. On the other hand, a western blot analysis showed no changes in 5-HT2A receptor immunoreactivity in the prefrontal cortex in either treated with antipsychotics or not treated schizophrenia patients but a decrease in mRNA level was found only in patients treated with antipsychotics [47]. The above findings indicate that the level of the 5-HT2A receptor in schizophrenia might depend on antipsychotic therapy but also on methods used in studies. Thus, the role of the 5-HT2A receptor in schizophrenia pathomechanism is not definitively defined yet.
Schizophrenia-like effects of psychedelics
Psychedelics induce schizophrenia-like related symptoms i.e., psychosis, sensorimotor gating impairments, and working memory deficits (Table 1).
Psychosis
Psychosis is a clinical syndrome represented by several symptoms i.e., delusions, hallucinations, and thought disorder [48]. Psychosis is a common feature of both psychedelic actions in normal volunteers and schizophrenia patients.
Psychedelics induce a psychosis-like syndrome in normal volunteers that is characterized by ego disturbances, illusions and hallucinations, thought disorders, paranoid thinking, and alterations in mood and affect [4, 5, 49]. Several psychotic symptoms induced by psychedelics are similar to the positive symptoms observed in the initial stages of acute schizophrenia episodes. Hallucinations and the loss of self-control over thought processes after the administration of psychedelics are similar to those experienced in acute psychosis in schizophrenia. Moreover, visual hallucinations typical for psychedelics are more often in acute than chronic schizophrenia [4]. However, hallucinations observed either in healthy volunteers using psychedelics or in patients with schizophrenia differ in many respects. Hallucinations induced by psychedelics are mostly visual, and both elementary (brightly colored geometric form figures) and complex (images of scenes and landscapes) with ordinary (humans, animals, and artifacts) and extraordinary entities (chimeras, spirits, and aliens). In schizophrenia, hallucinations mostly auditory, and visual hallucinations often include life-size images (faces, people, objects, events), and they are real, detailed, and anchored in space. In both cases, hallucinations have strong existential/metaphysical meanings. Usually, reality assessment is not impaired in people using hallucinogens, and they can distinguish between psychedelic effects and normal consciousness. In contrast, patients with schizophrenia have poor reality monitoring and insight. The duration of psychotic episodes is also different. Psychedelics induce transient psychotic episodes lasting a few hours unlike in schizophrenia patients, where the psychotic episodes are recurrent, and can last weeks or months [50].
Neuronal activity during psychosis was examined using positron emission tomography (PET) and [F-18]-fluorodeoxyglucose (FDG) that can analyze the organization of the human brain with neuronal function [51]. The results of the studies showed metabolic hyperfrontality during psychotic episodes in healthy volunteers using psychedelics (psilocybin, mescaline) [49, 52]. Hyperfrontal metabolic pattern was also associated with positive psychotic symptoms in acute unmedicated first-episode schizophrenia [53, 54] and also in unmedicated and medicated chronic patients with acute psychotic episodes [55, 56]. Thus, hyperfrontality metabolic changes are associated with acute psychotic episodes in schizophrenics and they are also induced by psychedelics, with contrasts to hypofrontality observed in patients with chronic schizophrenia [57,58,59].
Sensorimotor gating
Sensorimotor gating ability regulates sensory information that is transmitted to motor output systems. Sensory information processed centrally requires some degree of filtering or gating before accessing and impacting motor output [60]. Prepulse inhibition (PPI) of the acoustic startle response has been established as an operational measure of sensorimotor gating, and the PPI level may indicate the current integrity of sensorimotor gating mechanisms. PPI occurs when a relatively weak sensory stimulus (prepulse) is presented 30–500 ms before a strong startle-induced stimulus (pulse), and reduces the magnitude of the startle response. The fundamental mechanism initiating this inhibition is thought to resemble the normal process of filtering incoming sensory stimuli [61]. In humans, startle is measured from the eye blink response through electromyographic recordings from the orbicularis oculi muscle, and startle to acoustic stimuli or tactile stimuli are used [60]. PPI deficits are observed in schizophrenia patients [61,62,63], their unaffected relatives [64], and patients with schizotypal personality disorder [65].
Sensorimotor gating functions are modulated by several neurotransmitter systems i.e., dopaminergic, glutaminergic, serotonergic, γ-aminobutyric acid (GABA)-ergic, and cholinergic acting in cortical, limbic, striatal and brainstem structures [61, 66]. Some studies indicate that, among others, 5-HT2A serotonin receptors are involved in proper sensorimotor gating and 5-HT2A receptor polymorphisms might contribute to the PPI deficits in schizophrenia [67, 68]. Analysis of 5HT2A receptor polymorphism (A-1438G, T102C, and H452Y) showed that patients carrying the T102CTT and the A-1438GAA allele demonstrated higher PPI levels than all other variants. Carriers of the T102C-C/A-1438G-G allele exhibited a significantly lower PPI than patients homozygous for the T102C-T/A-1438G-A allele. On the other hand, the 5-HT2A receptor H452Y polymorphism did not affect startle parameters [68]. Another evidence indicates that epigenetic modifications (DNA methylation) of the 5-HT2A receptor allele are also involved in 5-HT2A receptor regulation, especially in an early stage of disease onset [67]. Thus, genetic background and epigenetic mechanism might implicate 5-HT2A receptor function in sensorimotor gating.
Hallucinogens are postulated to work by disrupting sensory filtering mechanisms, resulting in sensory overload and cognitive dysfunction [4, 69]. The effects of psychedelics on PPI were analyzed in humans and obtained results revealed diverse effects of psychedelic substances on PPI. DMT did not affect PPI at any using interstimulus intervals (ISI) [70]. In the case of psilocybin, some studies show an increase in PPI at 100 ms ISI [71], while others indicate that the effect of psilocybin on PPI was dependent on ISI. Psilocybin reduced PPI at short (30 ms), had no effect at medium (60 ms), and increased PPI at long (120–2000 ms) intervals [72]. On the other hand, LSD did not demonstrate a similar to psilocybin impact on PPI and decreased PPI at 30 ms, 60 ms, and 120 ms ISI [73]. Thus, hallucinogens alter PPI in humans, but the effects depend on the used substance, and ISI applied in the studies.
Working memory
Working memory is a limited-capacity, active short-term memory system that maintains information to guide and control behavior [74]. Working memory deficits are a permanent cognitive feature of schizophrenia and memory impairments are present during the prodromal stage and persist throughout schizophrenia [74]. Spatial working memory deficits were observed in schizophrenic patients in a spatial delayed-response task (DRT) [75]. Working memory was also studied in the Sternberg Item Recognition Test (SIRT), and functional magnetic resonance imaging (fMRI) showed that memory impairment in schizophrenic patients was related to a reduction in information processing efficiency, especially in the dorsal prefrontal cortex, or lack of an increase fMRI activation during task presentation was leading to performance impairments [76].
Functional genetic variants have a specific impact on cognitive abilities in a normal population. A study of two polymorphisms (rs6313 and rs4941573) in the 5-HT2A receptor showed that rs4941573 was associated with spatial working memory [77]. Some studies indicate that polymorphism in the T102C and A-1438G loci of the 5HT2A receptor is correlated with working memory impairment in schizophrenia patients, and T102C CC and A-1438G GG homozygotes have worse performance in working memory tasks [78].
Working memory was also examined in healthy participants using hallucinogens acting by 5-HT2A receptor (psilocybin and LSD). Psilocybin (0.25 mg/kg) induced spatial memory deficits in humans as measured by DRT. These deficits were linked to the activation of 5-HT2A, but not D2 receptors [5]. Another finding reported that psilocybin (215 μg/kg) reduced attentional tracking ability analyzed in a multi-object tracking test but did not affect spatial working memory measured in the Spatial Span test from Cambridge Neuropsychological Test Automated Battery (CANTAB) [79]. Ketanserin did not block the effect of psilocybin on attentional performance suggesting an involvement of the serotonin 5-HT1A receptor in the observed deficits [79]. The effect of psilocybin in medium 115 μg and high 250 μg/kg dose conditions on spatial working memory in the Spatial Span test was also examined by others [80]. Significant impairments in performance on the task were observed in the high-dose condition but not in the medium-dose condition. Working memory was also assessed with the letter N-back task from Penn Computerized Neurocognitive Battery, and psilocybin (10, 20, and 30 mg/70 kg) dose-dependent impaired the performance in the N-back test [81]. The above results indicate that the effect of psilocybin on working memory might be dependent on the dose used in the studies. Another psychedelic, LSD (100 μg) impaired working memory examined by Intra/Extra-Dimensional shift task (IED) and Spatial Working Memory task (SWM) from CANTAB [82]. Ayahuasca users also showed impairments in working memory performance tested in the Stenberg working memory task, and the Tower of London task [83].
Recent findings indicate that polymorphism of the 5-HT2A receptor as well as activation of the 5-HT2A receptor by psychedelics might induce the disruption of working memory that is observed in schizophrenia patients.
Glutamatergic transmission in psychedelics action
Numerous animal studies showed that the effects of psychedelics might be related to other than serotonergic transmission (Table 1). Early findings indicate that immediate-early genes (IEGs), i.e., c-Fos, Arc proteins induced by psychedelics, i.e., DOI, are not expressed in the 5-HT2A-positive cells [9, 84, 85]. The observed results of DOI action were directly relatable to the activation of 5-HT2A, but not the 5-HT2C receptors [84, 85]. Later studies showed that psychedelics (LSD and DOI) evoked c-Fos expression is present in cells positive for 5-HT2A mRNA [20], but the lack of 5-HT2A immunoreactivity in cells demonstrating DOI-stimulated IEGs transcription might suggest an indirect effect of hallucinogens on IEGs activation.
Dysregulation of glutamatergic signaling is indicated to be involved in schizophrenia pathogenesis [40], and some evidence also implies that glutaminergic transmission is involved in the hallucinogenic actions following 5-HT2A receptor activation. A recent study showed that psilocybin increases glutamate release in the rat frontal cortex [86] and human medial prefrontal cortex [87]. Other findings indicated that either an antagonist of the AMPA receptor, GYKI 52466, or an antagonist of the NMDA glutamate receptor, MK-801, attenuated an increase in IEGs expression (Arc, c-Fos) induced by DOI [84, 85]. The above results imply that ionotropic receptor activation, i.e., NMDA, and AMPA, is necessary for transcriptional activities induced by psychedelics. Moreover, a recent study revealed that psilocybin increased the level of NMDA receptor subunits, mainly GluN2B, but it did not affect the protein of AMPA receptor subunits (GluA1, GluA2) in the rat frontal cortex [86]. The above findings indicate that ionotropic glutamate receptors might play an essential role in the action of psychedelics.
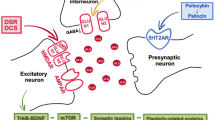
Electrophysiological studies revealed that enhancement of 5-HT2A receptor-induced glutamatergic transmission in the prefrontal cortex was suppressed by activation of glutamatergic metabotropic receptor mGlu2/3 [88, 89] and an agonist of mGlu2/3 also reduced DOI-induced c-Fos expression [89]. The above findings suggest that the activation of presynaptic mGlu2 autoreceptors blocked the stimulation of glutamatergic transmission induced by hallucinogenic 5-HT2A receptor agonists [90]. Another evidence implies that crosstalk between 5-HT2A and mGlu2 receptors is related to their presence in the postsynaptic part of cortical pyramidal neurons. The G-protein coupled receptors (GPCRs) studies in mouse and human cortex showed that Gq11-coupled 5-HT2A and the Gi/o -coupled mGlu2 receptor form a specific GPCR heteromeric complex [20, 91, 92]. 5-HT2A-mGlu2 receptor complex seems to be essential for the hallucinogenic-like behaviors caused by 5HT2A agonists i.e., head-twitch in animals. 5-HT2A receptor agonist (DOI, LSD) did not induce head-twitch behavior in mGlu2 knockout mice [93]. Moreover, behavioral responses of 5-HT2A receptor activation were returned in mice with overexpression mGlu2 in the frontal cortex using a viral (HSV)-mediated transgene expression method [92].
The results from animal studies have great translational potential since postmortem analysis of the frontal cortex of schizophrenic subjects revealed the changes in the expression of 5-HT2A and mGlu2 receptors [46, 92, 94]. Moreover, functional crosstalk between 5-HT2A and mGlu2 receptors was also found in schizophrenia because the activation of Gq11 signaling by mGlu2 receptor agonist was dysregulated in the postmortem frontal cortex of schizophrenic subjects [94]. Thus, an interaction between 5-HT2A and mGlu2 receptors could be a relevant explanation of the mechanism involved in psychosis and schizophrenia treatment [95].
5-HT2A receptor as a target in schizophrenia treatment
5-HT2A receptor antagonists
Antipsychotics are a group of drugs used in schizophrenia therapy. They are divided based on data of release (first vs. second generation), efficacy, and side effects. However, all antipsychotics share a blockade of the D2 receptor as a necessary mechanism for their action [96]. They are predominantly useful in positive symptoms of schizophrenia and have serious limitations in therapy including low effectiveness for negative symptoms and cognitive deficits, and side effects (for example weight gain, metabolic disturbances, extrapyramidal symptoms (EPS), akathisia, hyperprolactinemia, and sleep disturbances) [97].
First-generation antipsychotics (chlorpromazine and haloperidol) are characterized by their relatively high-affinity antagonism at the D2 receptor, and they are efficacious on positive symptoms of schizophrenia. In contrast, most second-generation antipsychotics (clozapine, risperidone, and olanzapine) have relatively lower D2 receptor antagonism but they possess relatively higher 5-HT2A receptor antagonism that is supposed to increase their efficacy in the treatment of negative and cognitive abnormalities in schizophrenia [97]. Recently, a new antipsychotic, lumateperone has been approved by the Drug and Food Administration (FDA). Lumateperone is an antagonist of 5-HT2A and postsynaptic D2 receptors but also acts as a partial agonist of the presynaptic dopamine D2 receptor. Similar to other second-generation antipsychotics, it has a higher affinity for the 5-HT2A receptor compared to the D2 receptor. Clinical studies reveal that lumateperone significantly reduces positive and negative schizophrenia symptoms and does not present EPS and metabolic adverse effects usually observed in antipsychotic treatment [98, 99]. Thus, lumateperone seems to be a promising option for schizophrenia therapy in patients with metabolic dysfunction and intolerance to EPS.
A meta-analysis study indicated that 5-HT2A antagonists could be useful in the treatment of negative symptoms of schizophrenia [100]. Recent findings also suggest that the antipsychotic affinity for the 5-HT2A receptor is not significantly associated with specific side effects i.e., sedation, EPS, prolactin increase, and weight gain [101].
Clinical use of selective antagonists of the 5-HT2A receptor in schizophrenia pharmacotherapy has not been approved by FDA. 5-HT2A antagonists, such as roluperidone (MIN-101), eplivanserin (SR46349B), fananserin, and ritanserin, were checked in clinical trials, however, their effectiveness in schizophrenia treatment was not satisfactory [97]. At this moment, there are no FDA-approved non-dopaminergic antipsychotics for schizophrenia therapy. On the other hand, pimavanserin, a reverse agonist of the 5-HT2A receptor with affinity to 5-HT2C but not the D2 receptor, is used in the treatment of hallucinations and delusions associated with Parkinson’s disease psychosis [97]. Moreover, combined treatment of pimavanserin with a subeffective dose of risperidone increased the therapeutic effects and enhanced the safety of risperidone treatment in schizophrenia [102]. Thus, the 5-HT2A receptor might be a promising target in schizophrenia pharmacotherapy but an affinity and antagonism only to the 5-HT2A receptor are not sufficient to get full antipsychotic effects.
5-HT2A receptor agonists
Hallucinogenic properties of 5-HT2A agonists exclude schizophrenia from the current list of potential therapeutic targets of psychedelics. Given the historical background of the administration of psychedelics to schizophrenia patients in the 1950s and 1960s showing their psychotomimetic and hallucinogenic properties [103, 104], their clinical applications require extreme caution. However, in some studies from the 1950s and 1960s, psychedelic administration to young people with autism and schizophrenia improved symptoms domain called today as negative symptoms. What is more, exacerbation of psychosis was not always observed [105, 106].
At present, people with a personal family history of psychosis are generally excluded from clinical trials of psychedelic compounds [107]. However, there are some presumptions that 5-HT2A agonists might be worthy of consideration in schizophrenia therapy, especially when negative symptoms and cognitive deficits are predominant in the features of the disorder [108], and these deficits are associated with structural brain changes, i.e., significant cortical thinning [109,110,111], reduction gray matter volume [112], reduction in synaptic proteins and synaptic loss [33, 113]. In the context of the above neuroanatomical changes observed in schizophrenia, the 5-HT2A receptor agonist's ability to produce rapid and long-lasting changes in neural plasticity might be essential from the perspective of schizophrenia therapy. Several findings indicate that psychedelics (DOI, psilocybin, LSD, DMT) increase dendritic arbor complexity, promote dendritic spine growth and stimulate synapse formation [114,115,116,117,118]. The above alterations are related to the neurotrophic effects of psychedelics. They increase neurotrophic factor levels, i.e., brain-derived neurotrophic factor (BDNF) and activate its receptor TrkB [115, 119,120,121,122]. Neurotrophin levels are impaired in schizophrenia and antipsychotic therapy has diverse effects on their level [123]. Thus, psychedelic treatment might have positive outcomes on synapse loss and cognitive deficits observed in schizophrenia by normalizing neurotrophin levels.
Evidence indicates that the window of neuroplastic effects for psychedelics is between a few hours to a few days, although some neuroplasticity alterations might be observed for at least a month [124]. Psychedelics also induce long-lasting transcriptomic and epigenomic changes, and most of them are related to genes associated with synaptic plasticity (axonogenesis, synapse organization, synapse assembly). However, some of them are correlated with genetic risk for schizophrenia development (Cntnap2, Nfasc, Drd2, Grin2b, Prkca, Slc1a2) [114, 125, 126].
The potential benefits of using 5-HT2A receptor agonists in schizophrenia treatment cause a discussion about strategies for avoiding the psychotogenic effects of psychedelics while maintaining neuroplastic effects. At the doses used in the therapy of psychiatric disorders, all psychedelic drugs induce the “trip”. The patients required observation up to 24 h after treatment [127]. Presently, the doses used in clinical studies with psilocybin are 20–25 mg/70 kg for adults selected to stimulate evidently, subjectively experienced effects on perception, cognition, and mood [107]. On the other hand, some findings indicate that psychedelics might be used in microdoses to get therapeutical effects [128, 129]. Psychodelic microdose is defined as 10% of the dose inducing psychedelic effects in the average adult, i.e., for psilocybin 2.0–2.5 mg [130]. Psychedelic microdosing could be operated in schizophrenia treatment without inducing psychotic effects. However, further studies will be needed to determine the appropriate dose and dosing regimen given therapeutic benefits in improving negative and cognitive symptoms with the absence of psychotogenic effects, and also tolerability of treatment [107].
Pharmacological studies showed that some 5-HT2A agonists do not possess hallucinogenic properties [131] and they have different transcriptome fingerprints [22]. Moreover, some evidence indicates that non-hallucinogenic psychedelic analogs might be a therapeutic option because of their ability to induce neuroplastic effects without a trip [132,133,134]. However, more studies are needed to verify their usefulness in the treatment of negative or cognitive schizophrenia dysfunction.
Thus, psychedelic microdosing or non-hallucinogenic agonist administration might be considered an alternative therapeutic option in schizophrenia treatment. However, at present 5-HT2A agonists are not used for treatment of patients with schizophrenia.
Summary and conclusions
The studies of using psychedelics in clinical therapy of psychiatric disorders started in the 1950s and 1960s but revealed that psychedelic behavioral effects shared some similarities with schizophrenia symptoms. Subsequent biochemical studies in animal models indicated that there is a certain likeness between psychedelics and schizophrenia pathophysiological background, including 5-HT2A receptor polymorphism in schizophrenia, and glutamatergic transmission dysfunction in both cases. However, in the case of psychedelics, an interaction between 5-HT2A and mGlu2 receptors seems to play an important role in the hallucinogen's appearance, in schizophrenia hypofunction of the NMDA receptor is suggested. At present, there is a lot of data showing an opportunity to use psychedelics in therapy for several psychiatric disorders (depression or posttraumatic stress disorders), but their use in schizophrenia treatment is still uncertain. The neuroplastic effects of hallucinogens and non-hallucinogens 5-HT2A agonists would be of interest in the treatment of negative and cognitive deficits in schizophrenia, and the safest option would be to use non-hallucinogenic ones. Currently, there is not enough data to predict the further application of 5-HT2A agonists in schizophrenia treatment, although it might be a beneficial target for new therapies. Additional preclinical and clinical investigations are necessary to confirm the effectiveness of psychedelics in schizophrenia treatment.
Data availability
Data sharing is not applicable for this article as no datasets were generated or analyzed during the current study.
Abbreviations
- 5-HT2A :
-
Serotonin 5-hydroxytryptamine receptor
- AMPA:
-
α-Amino-3-hydroxy-5-methyl-4-isoxazolepropionic receptor
- ASC:
-
Altered states of consciousness
- APZ:
-
Abnormal Mental States
- DMT:
-
N, N-Dimethyltryptamine
- DOI:
-
2,5-Dimethoxy-4-iodoamphetamine
- HRS:
-
Hallucinogen rating scale
- IEG:
-
Immediate-early gene
- LSD:
-
Lysergic acid diethylamide
- mGlu:
-
Metabotropic receptor
- NMDA:
-
N-Methyl-d-aspartate receptor
- PPI:
-
Prepulse inhibition of the acoustic startle response
References
Vollenweider FX. Brain mechanisms of hallucinogens and entactogens. Dialogues Clin Neurosci. 2001;3:265–79.
Inserra A, De Gregorio D, Gobbi G. Psychedelics in psychiatry: neuroplastic, immunomodulatory, and neurotransmitter mechanisms. Pharmacol Rev. 2021;73:202–77.
Nichols DE. Psychedelics. Pharmacol Rev. 2016;68:264–355.
Vollenweider FX, Geyer MA. A systems model of altered consciousness: integrating natural and drug-induced psychoses. Brain Res Bull. 2001;56:495–507.
Vollenweider FX, Vollenweider-Scherpenhuyzen MF, Babler A, Vogel H, Hell D. Psilocybin induces schizophrenia-like psychosis in humans via a serotonin-2 agonist action. NeuroReport. 1998;9:3897–902.
Madsen MK, Fisher PM, Burmester D, Dyssegaard A, Stenbaek DS, Kristiansen S, et al. Psychedelic effects of psilocybin correlate with serotonin 2A receptor occupancy and plasma psilocin levels. Neuropsychopharmacology. 2019;44:1328–34.
Jakab RL, Goldman-Rakic PS. 5-Hydroxytryptamine2A serotonin receptors in the primate cerebral cortex: possible site of action of hallucinogenic and antipsychotic drugs in pyramidal cell apical dendrites. Proc Natl Acad Sci USA. 1998;95:735–40.
Lopez-Gimenez JF, Mengod G, Palacios JM, Vilaro MT. Selective visualization of rat brain 5-HT2A receptors by autoradiography with [3H]MDL 100,907. Naunyn Schmiedebergs Arch Pharmacol. 1997;356:446–54.
Mackowiak M, Chocyk A, Fijal K, Czyrak A, Wedzony K. c-Fos proteins, induced by the serotonin receptor agonist DOI, are not expressed in 5-HT2A positive cortical neurons. Brain Res Mol Brain Res. 1999;71:358–63.
Barnes NM, Sharp T. A review of central 5-HT receptors and their function. Neuropharmacology. 1999;38:1083–152.
Hannon J, Hoyer D. Molecular biology of 5-HT receptors. Behav Brain Res. 2008;195:198–213.
Nichols DE, Nichols CD. Serotonin receptors. Chem Rev. 2008;108:1614–41.
Raymond JR, Mukhin YV, Gelasco A, Turner J, Collinsworth G, Gettys TW, et al. Multiplicity of mechanisms of serotonin receptor signal transduction. Pharmacol Ther. 2001;92:179–212.
Berg KA, Maayani S, Goldfarb J, Scaramellini C, Leff P, Clarke WP. Effector pathway-dependent relative efficacy at serotonin type 2A and 2C receptors: evidence for agonist-directed trafficking of receptor stimulus. Mol Pharmacol. 1998;54:94–104.
Felder CC, Kanterman RY, Ma AL, Axelrod J. Serotonin stimulates phospholipase A2 and the release of arachidonic acid in hippocampal neurons by a type 2 serotonin receptor that is independent of inositolphospholipid hydrolysis. Proc Natl Acad Sci USA. 1990;87:2187–91.
Kurrasch-Orbaugh DM, Watts VJ, Barker EL, Nichols DE. Serotonin 5-hydroxytryptamine 2A receptor-coupled phospholipase C and phospholipase A2 signaling pathways have different receptor reserves. J Pharmacol Exp Ther. 2003;304:229–37.
Kurrasch-Orbaugh DM, Parrish JC, Watts VJ, Nichols DE. A complex signaling cascade links the serotonin2A receptor to phospholipase A2 activation: the involvement of MAP kinases. J Neurochem. 2003;86:980–91.
Schmid CL, Bohn LM. Serotonin, but not N-methyltryptamines, activates the serotonin 2A receptor via a ss-arrestin2/Src/Akt signaling complex in vivo. J Neurosci. 2010;30:13513–24.
Barclay Z, Dickson L, Robertson DN, Johnson MS, Holland PJ, Rosie R, et al. 5-HT2A receptor signalling through phospholipase D1 associated with its C-terminal tail. Biochem J. 2011;436:651–60.
Gonzalez-Maeso J, Weisstaub NV, Zhou M, Chan P, Ivic L, Ang R, et al. Hallucinogens recruit specific cortical 5-HT(2A) receptor-mediated signaling pathways to affect behavior. Neuron. 2007;53:439–52.
Karaki S, Becamel C, Murat S, Mannoury la Cour C, Millan MJ, Prezeau L, et al. Quantitative phosphoproteomics unravels biased phosphorylation of serotonin 2A receptor at Ser280 by hallucinogenic versus nonhallucinogenic agonists. Mol Cell Proteomics. 2014; 13: 1273–85.
Gonzalez-Maeso J, Yuen T, Ebersole BJ, Wurmbach E, Lira A, Zhou M, et al. Transcriptome fingerprints distinguish hallucinogenic and nonhallucinogenic 5-hydroxytryptamine 2A receptor agonist effects in mouse somatosensory cortex. J Neurosci. 2003;23:8836–43.
Liu X, Zhu H, Gao H, Tian X, Tan B, Su R. G(s) signaling pathway distinguishes hallucinogenic and nonhallucinogenic 5-HT(2A)R agonists induced head twitch response in mice. Biochem Biophys Res Commun. 2022;598:20–5.
Correll CU, Schooler NR. Negative symptoms in schizophrenia: a review and clinical guide for recognition, assessment, and treatment. Neuropsychiatr Dis Treat. 2020;16:519–34.
Keefe RS, Fox KH, Harvey PD, Cucchiaro J, Siu C, Loebel A. Characteristics of the MATRICS Consensus Cognitive Battery in a 29-site antipsychotic schizophrenia clinical trial. Schizophr Res. 2011;125:161–8.
McCutcheon RA, Reis Marques T, Howes OD. Schizophrenia—an overview. JAMA Psychiat. 2020;77:201–10.
Messias EL, Chen CY, Eaton WW. Epidemiology of schizophrenia: review of findings and myths. Psychiatr Clin North Am. 2007;30:323–38.
Carrion RE, Correll CU, Auther AM, Cornblatt BA. A severity-based clinical staging model for the psychosis prodrome: longitudinal findings from the new york recognition and prevention program. Schizophr Bull. 2017;43:64–74.
George M, Maheshwari S, Chandran S, Manohar JS, Sathyanarayana Rao TS. Understanding the schizophrenia prodrome. Indian J Psychiatry. 2017;59:505–9.
Jaaro-Peled H, Sawa A. Neurodevelopmental factors in schizophrenia. Psychiatr Clin North Am. 2020;43:263–74.
Wawrzczak-Bargiela A, Bilecki W, Mackowiak M. Epigenetic targets in schizophrenia development and therapy. Brain Sci. 2023;13:426.
Mirnics K, Middleton FA, Marquez A, Lewis DA, Levitt P. Molecular characterization of schizophrenia viewed by microarray analysis of gene expression in prefrontal cortex. Neuron. 2000;28:53–67.
Osimo EF, Beck K, Reis Marques T, Howes OD. Synaptic loss in schizophrenia: a meta-analysis and systematic review of synaptic protein and mRNA measures. Mol Psychiatry. 2019;24:549–61.
Radhakrishnan R, Skosnik PD, Ranganathan M, Naganawa M, Toyonaga T, Finnema S, et al. In vivo evidence of lower synaptic vesicle density in schizophrenia. Mol Psychiatry. 2021;26:7690–8.
Selemon LD, Goldman-Rakic PS. The reduced neuropil hypothesis: a circuit based model of schizophrenia. Biol Psychiatry. 1999;45:17–25.
Thune JJ, Uylings HB, Pakkenberg B. No deficit in total number of neurons in the prefrontal cortex in schizophrenics. J Psychiatr Res. 2001;35:15–21.
Bennett MR. Synapse formation and regression in the cortex during adolescence and in schizophrenia. Med J Aust. 2009;190:S14–6.
Howes OD, Shatalina E. Integrating the Neurodevelopmental and Dopamine Hypotheses of Schizophrenia and the role of cortical excitation-inhibition balance. Biol Psychiatry. 2022;92:501–13.
Bilecki W, Mackowiak M. Gene expression and epigenetic regulation in the prefrontal cortex of schizophrenia. Genes (Basel). 2023;14:243.
Uno Y, Coyle JT. Glutamate hypothesis in schizophrenia. Psychiatry Clin Neurosci. 2019;73:204–15.
Yang AC, Tsai SJ. New targets for schizophrenia treatment beyond the dopamine hypothesis. Int J Mol Sci. 2017;18:1689.
Egerton A, Grace AA, Stone J, Bossong MG, Sand M, McGuire P. Glutamate in schizophrenia: neurodevelopmental perspectives and drug development. Schizophr Res. 2020;223:59–70.
Stepnicki P, Kondej M, Kaczor AA. Current concepts and treatments of schizophrenia. Molecules. 2018;23:2087.
Stahl SM. Beyond the dopamine hypothesis of schizophrenia to three neural networks of psychosis: dopamine, serotonin, and glutamate. CNS Spectr. 2018;23:187–91.
Kang K, Huang XF, Wang Q, Deng C. Decreased density of serotonin 2A receptors in the superior temporal gyrus in schizophrenia–a postmortem study. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:867–71.
Muguruza C, Moreno JL, Umali A, Callado LF, Meana JJ, Gonzalez-Maeso J. Dysregulated 5-HT(2A) receptor binding in postmortem frontal cortex of schizophrenic subjects. Eur Neuropsychopharmacol. 2013;23:852–64.
Garcia-Bea A, Miranda-Azpiazu P, Muguruza C, Marmolejo-Martinez-Artesero S, Diez-Alarcia R, Gabilondo AM, et al. Serotonin 5-HT(2A) receptor expression and functionality in postmortem frontal cortex of subjects with schizophrenia: selective biased agonism via G(alphai1)-proteins. Eur Neuropsychopharmacol. 2019;29:1453–63.
Gaebel W, Zielasek J. Focus on psychosis. Dial Clin Neurosci. 2015;17:9–18.
Vollenweider FX, Leenders KL, Scharfetter C, Maguire P, Stadelmann O, Angst J. Positron emission tomography and fluorodeoxyglucose studies of metabolic hyperfrontality and psychopathology in the psilocybin model of psychosis. Neuropsychopharmacology. 1997;16:357–72.
Leptourgos P, Fortier-Davy M, Carhart-Harris R, Corlett PR, Dupuis D, Halberstadt AL, et al. Hallucinations under psychedelics and in the schizophrenia spectrum: an interdisciplinary and multiscale comparison. Schizophr Bull. 2020;46:1396–408.
Phelps ME, Huang SC, Hoffman EJ, Selin C, Sokoloff L, Kuhl DE. Tomographic measurement of local cerebral glucose metabolic rate in humans with (F-18)2-fluoro-2-deoxy-d-glucose: validation of method. Ann Neurol. 1979;6:371–88.
Hermle L, Funfgeld M, Oepen G, Botsch H, Borchardt D, Gouzoulis E, et al. Mescaline-induced psychopathological, neuropsychological, and neurometabolic effects in normal subjects: experimental psychosis as a tool for psychiatric research. Biol Psychiatry. 1992;32:976–91.
Cleghorn JM, Garnett ES, Nahmias C, Firnau G, Brown GM, Kaplan R, et al. Increased frontal and reduced parietal glucose metabolism in acute untreated schizophrenia. Psychiatry Res. 1989;28:119–33.
Kaplan RD, Szechtman H, Franco S, Szechtman B, Nahmias C, Garnett ES, et al. Three clinical syndromes of schizophrenia in untreated subjects: relation to brain glucose activity measured by positron emission tomography (PET). Schizophr Res. 1993;11:47–54.
Ebmeier KP, Blackwood DH, Murray C, Souza V, Walker M, Dougall N, et al. Single-photon emission computed tomography with 99mTc-exametazime in unmedicated schizophrenic patients. Biol Psychiatry. 1993;33:487–95.
Liddle PF, Friston KJ, Frith CD, Hirsch SR, Jones T, Frackowiak RS. Patterns of cerebral blood flow in schizophrenia. Br J Psychiatry. 1992;160:179–86.
Hazlett EA, Buchsbaum MS, Jeu LA, Nenadic I, Fleischman MB, Shihabuddin L, et al. Hypofrontality in unmedicated schizophrenia patients studied with PET during performance of a serial verbal learning task. Schizophr Res. 2000;43:33–46.
Hill K, Mann L, Laws KR, Stephenson CM, Nimmo-Smith I, McKenna PJ. Hypofrontality in schizophrenia: a meta-analysis of functional imaging studies. Acta Psychiatr Scand. 2004;110:243–56.
Wolkin A, Sanfilipo M, Wolf AP, Angrist B, Brodie JD, Rotrosen J. Negative symptoms and hypofrontality in chronic schizophrenia. Arch Gen Psychiatry. 1992;49:959–65.
Powell SB, Weber M, Geyer MA. Genetic models of sensorimotor gating: relevance to neuropsychiatric disorders. Curr Top Behav Neurosci. 2012;12:251–318.
Braff DL, Geyer MA, Swerdlow NR. Human studies of prepulse inhibition of startle: normal subjects, patient groups, and pharmacological studies. Psychopharmacology. 2001;156:234–58.
Kumari V, Fannon D, Geyer MA, Premkumar P, Antonova E, Simmons A, et al. Cortical grey matter volume and sensorimotor gating in schizophrenia. Cortex. 2008;44:1206–14.
Swerdlow NR, Weber M, Qu Y, Light GA, Braff DL. Realistic expectations of prepulse inhibition in translational models for schizophrenia research. Psychopharmacology. 2008;199:331–88.
Cadenhead KS, Swerdlow NR, Shafer KM, Diaz M, Braff DL. Modulation of the startle response and startle laterality in relatives of schizophrenic patients and in subjects with schizotypal personality disorder: evidence of inhibitory deficits. Am J Psychiatry. 2000;157:1660–8.
Cadenhead KS, Geyer MA, Braff DL. Impaired startle prepulse inhibition and habituation in patients with schizotypal personality disorder. Am J Psychiatry. 1993;150:1862–7.
Swerdlow NR, Braff DL, Geyer MA. Sensorimotor gating of the startle reflex: what we said 25 years ago, what has happened since then, and what comes next. J Psychopharmacol. 2016;30:1072–81.
Abdolmaleky HM, Yaqubi S, Papageorgis P, Lambert AW, Ozturk S, Sivaraman V, et al. Epigenetic dysregulation of HTR2A in the brain of patients with schizophrenia and bipolar disorder. Schizophr Res. 2011;129:183–90.
Quednow BB, Kuhn KU, Mossner R, Schwab SG, Schuhmacher A, Maier W, et al. Sensorimotor gating of schizophrenia patients is influenced by 5-HT2A receptor polymorphisms. Biol Psychiatry. 2008;64:434–7.
Riba J, Rodriguez-Fornells A, Barbanoj MJ. Effects of ayahuasca on sensory and sensorimotor gating in humans as measured by P50 suppression and prepulse inhibition of the startle reflex, respectively. Psychopharmacology. 2002;165:18–28.
Heekeren K, Neukirch A, Daumann J, Stoll M, Obradovic M, Kovar KA, et al. Prepulse inhibition of the startle reflex and its attentional modulation in the human S-ketamine and N, N-dimethyltryptamine (DMT) models of psychosis. J Psychopharmacol. 2007;21:312–20.
Gouzoulis-Mayfrank E, Heekeren K, Thelen B, Lindenblatt H, Kovar KA, Sass H, et al. Effects of the hallucinogen psilocybin on habituation and prepulse inhibition of the startle reflex in humans. Behav Pharmacol. 1998;9:561–6.
Vollenweider FX, Csomor PA, Knappe B, Geyer MA, Quednow BB. The effects of the preferential 5-HT2A agonist psilocybin on prepulse inhibition of startle in healthy human volunteers depend on interstimulus interval. Neuropsychopharmacology. 2007;32:1876–87.
Schmid Y, Enzler F, Gasser P, Grouzmann E, Preller KH, Vollenweider FX, et al. Acute effects of lysergic acid diethylamide in healthy subjects. Biol Psychiatry. 2015;78:544–53.
Ichinose M, Park S. Mechanisms underlying visuospatial working memory impairments in schizophrenia. Curr Top Behav Neurosci. 2019;41:345–67.
Park S, Holzman PS. Schizophrenics show spatial working memory deficits. Arch Gen Psychiatry. 1992;49:975–82.
Metzak PD, Riley JD, Wang L, Whitman JC, Ngan ET, Woodward TS. Decreased efficiency of task-positive and task-negative networks during working memory in schizophrenia. Schizophr Bull. 2012;38:803–13.
Gong P, Li J, Wang J, Lei X, Chen D, Zhang K, et al. Variations in 5-HT2A influence spatial cognitive abilities and working memory. Can J Neurol Sci. 2011;38:303–8.
Alfimova MV, Monakhov MV, Abramova LI, Golubev SA, Golimbet VE. Polymorphism of serotonin receptor genes (5-HTR2A) and Dysbindin (DTNBP1) and individual components of short-term verbal memory processes in Schizophrenia. Neurosci Behav Physiol. 2010;40:934–40.
Carter OL, Burr DC, Pettigrew JD, Wallis GM, Hasler F, Vollenweider FX. Using psilocybin to investigate the relationship between attention, working memory, and the serotonin 1A and 2A receptors. J Cogn Neurosci. 2005;17:1497–508.
Wittmann M, Carter O, Hasler F, Cahn BR, Grimberg U, Spring P, et al. Effects of psilocybin on time perception and temporal control of behaviour in humans. J Psychopharmacol. 2007;21:50–64.
Barrett FS, Carbonaro TM, Hurwitz E, Johnson MW, Griffiths RR. Double-blind comparison of the two hallucinogens psilocybin and dextromethorphan: effects on cognition. Psychopharmacology. 2018;235:2915–27.
Pokorny T, Duerler P, Seifritz E, Vollenweider FX, Preller KH. LSD acutely impairs working memory, executive functions, and cognitive flexibility, but not risk-based decision-making. Psychol Med. 2020;50:2255–64.
Bouso JC, Fabregas JM, Antonijoan RM, Rodriguez-Fornells A, Riba J. Acute effects of ayahuasca on neuropsychological performance: differences in executive function between experienced and occasional users. Psychopharmacology. 2013;230:415–24.
Pei Q, Tordera R, Sprakes M, Sharp T. Glutamate receptor activation is involved in 5-HT2 agonist-induced Arc gene expression in the rat cortex. Neuropharmacology. 2004;46:331–9.
Scruggs JL, Patel S, Bubser M, Deutch AY. DOI-Induced activation of the cortex: dependence on 5-HT2A heteroceptors on thalamocortical glutamatergic neurons. J Neurosci. 2000;20:8846–52.
Wojtas A, Bysiek A, Wawrzczak-Bargiela A, Szych Z, Majcher-Maslanka I, Herian M, et al. Effect of psilocybin and ketamine on brain neurotransmitters, glutamate receptors, DNA and rat behavior. Int J Mol Sci. 2022;23:6713.
Mason NL, Kuypers KPC, Muller F, Reckweg J, Tse DHY, Toennes SW, et al. Me, myself, bye: regional alterations in glutamate and the experience of ego dissolution with psilocybin. Neuropsychopharmacology. 2020;45:2003–11.
Marek GJ, Wright RA, Schoepp DD, Monn JA, Aghajanian GK. Physiological antagonism between 5-hydroxytryptamine(2A) and group II metabotropic glutamate receptors in prefrontal cortex. J Pharmacol Exp Ther. 2000;292:76–87.
Zhai Y, George CA, Zhai J, Nisenbaum ES, Johnson MP, Nisenbaum LK. Group II metabotropic glutamate receptor modulation of DOI-induced c-fos mRNA and excitatory responses in the cerebral cortex. Neuropsychopharmacology. 2003;28:45–52.
Aghajanian GK, Marek GJ. Serotonin model of schizophrenia: emerging role of glutamate mechanisms. Brain Res Brain Res Rev. 2000;31:302–12.
Fribourg M, Moreno JL, Holloway T, Provasi D, Baki L, Mahajan R, et al. Decoding the signaling of a GPCR heteromeric complex reveals a unifying mechanism of action of antipsychotic drugs. Cell. 2011;147:1011–23.
Moreno JL, Muguruza C, Umali A, Mortillo S, Holloway T, Pilar-Cuellar F, et al. Identification of three residues essential for 5-hydroxytryptamine 2A-metabotropic glutamate 2 (5-HT2A.mGlu2) receptor heteromerization and its psychoactive behavioral function. J Biol Chem. 2012;287:44301–19.
Moreno JL, Holloway T, Albizu L, Sealfon SC, Gonzalez-Maeso J. Metabotropic glutamate mGlu2 receptor is necessary for the pharmacological and behavioral effects induced by hallucinogenic 5-HT2A receptor agonists. Neurosci Lett. 2011;493:76–9.
Moreno JL, Miranda-Azpiazu P, Garcia-Bea A, Younkin J, Cui M, Kozlenkov A, et al. Allosteric signaling through an mGlu2 and 5-HT2A heteromeric receptor complex and its potential contribution to schizophrenia. Sci Signal. 2016;9:ra5.
Saha S, Gonzalez-Maeso J. The crosstalk between 5-HT(2A)R and mGluR2 in schizophrenia. Neuropharmacology. 2023;230: 109489.
Kapur S, Remington G. Dopamine D(2) receptors and their role in atypical antipsychotic action: still necessary and may even be sufficient. Biol Psychiatry. 2001;50:873–83.
Kantrowitz JT. Targeting serotonin 5-HT(2A) receptors to better treat schizophrenia: rationale and current approaches. CNS Drugs. 2020;34:947–59.
Edinoff A, Wu N, deBoisblanc C, Feltner CO, Norder M, Tzoneva V, et al. Lumateperone for the treatment of schizophrenia. Psychopharmacol Bull. 2020;50:32–59.
Greenwood J, Acharya RB, Marcellus V, Rey JA. Lumateperone: a novel antipsychotic for schizophrenia. Ann Pharmacother. 2021;55:98–104.
Romeo B, Willaime L, Rari E, Benyamina A, Martelli C. Efficacy of 5-HT2A antagonists on negative symptoms in patients with schizophrenia: a meta-analysis. Psychiatry Res. 2023;321: 115104.
Olten B, Bloch MH. Meta regression: relationship between antipsychotic receptor binding profiles and side-effects. Prog Neuropsychopharmacol Biol Psychiatry. 2018;84:272–81.
Meltzer HY, Elkis H, Vanover K, Weiner DM, van Kammen DP, Peters P, et al. Pimavanserin, a selective serotonin (5-HT)2A-inverse agonist, enhances the efficacy and safety of risperidone, 2 mg/day, but does not enhance efficacy of haloperidol, 2 mg/day: comparison with reference dose risperidone, 6 mg/day. Schizophr Res. 2012;141:144–52.
Cholden LS, Kurland A, Savage C. Clinical reactions and tolerance to LSD in chronic schizophrenia. J Nerv Ment Dis. 1955;122:211–21.
Gouzoulis-Mayfrank E, Hermle L, Thelen B, Sass H. History, rationale and potential of human experimental hallucinogenic drug research in psychiatry. Pharmacopsychiatry. 1998;31(Suppl 2):63–8.
Bender L. D-lysergic acid in the treatment of the biological features of childhood schizophrenia. Dis Nerv Syst. 1966;7(Suppl):43–6.
Mogar RE, Aldrich RW. The use of psychedelic agents with autistic schizophrenic children. Behav Neuropsychiatry. 1969;1:44–50.
Wolf G, Singh S, Blakolmer K, Lerer L, Lifschytz T, Heresco-Levy U, et al. Could psychedelic drugs have a role in the treatment of schizophrenia? Rationale and strategy for safe implementation. Mol Psychiatry. 2023;28:44–58.
Sawa A, Snyder SH. Schizophrenia: diverse approaches to a complex disease. Science. 2002;296:692–5.
Kubota M, Miyata J, Yoshida H, Hirao K, Fujiwara H, Kawada R, et al. Age-related cortical thinning in schizophrenia. Schizophr Res. 2011;125:21–9.
Narr KL, Toga AW, Szeszko P, Thompson PM, Woods RP, Robinson D, et al. Cortical thinning in cingulate and occipital cortices in first episode schizophrenia. Biol Psychiatry. 2005;58:32–40.
Yan J, Cui Y, Li Q, Tian L, Liu B, Jiang T, et al. Cortical thinning and flattening in schizophrenia and their unaffected parents. Neuropsychiatr Dis Treat. 2019;15:935–46.
Jiang Y, Luo C, Li X, Duan M, He H, Chen X, et al. Progressive reduction in gray matter in patients with schizophrenia assessed with MR Imaging by using causal network analysis. Radiology. 2018;287:633–42.
Glausier JR, Lewis DA. Dendritic spine pathology in schizophrenia. Neuroscience. 2013;251:90–107.
de la Fuente RM, Zhu B, Guevara CA, Naler LB, Saunders JM, Zhou Z, et al. Prolonged epigenomic and synaptic plasticity alterations following single exposure to a psychedelic in mice. Cell Rep. 2021;37: 109836.
Ly C, Greb AC, Cameron LP, Wong JM, Barragan EV, Wilson PC, et al. Psychedelics promote structural and functional neural plasticity. Cell Rep. 2018;23:3170–82.
Ly C, Greb AC, Vargas MV, Duim WC, Grodzki ACG, Lein PJ, et al. Transient stimulation with psychoplastogens is sufficient to initiate neuronal growth. ACS Pharmacol Transl Sci. 2021;4:452–60.
Raval NR, Johansen A, Donovan LL, Ros NF, Ozenne B, Hansen HD, et al. A single dose of psilocybin increases synaptic density and decreases 5-HT(2A) receptor density in the pig brain. Int J Mol Sci. 2021;22:835.
Shao LX, Liao C, Gregg I, Davoudian PA, Savalia NK, Delagarza K, et al. Psilocybin induces rapid and persistent growth of dendritic spines in frontal cortex in vivo. Neuron. 2021;109(2535–2544): e4.
de Almeida RN, Galvao ACM, da Silva FS, Silva E, Palhano-Fontes F, Maia-de-Oliveira JP, et al. Modulation of serum brain-derived neurotrophic factor by a single dose of ayahuasca: observation from a randomized controlled trial. Front Psychol. 2019;10:1234.
Holze F, Vizeli P, Ley L, Muller F, Dolder P, Stocker M, et al. Acute dose-dependent effects of lysergic acid diethylamide in a double-blind placebo-controlled study in healthy subjects. Neuropsychopharmacology. 2021;46:537–44.
Hutten N, Mason NL, Dolder PC, Theunissen EL, Holze F, Liechti ME, et al. Low doses of LSD acutely increase bdnf blood plasma levels in healthy volunteers. ACS Pharmacol Transl Sci. 2021;4:461–6.
Moliner R, Girych M, Brunello CA, Kovaleva V, Biojone C, Enkavi G, et al. Psychedelics promote plasticity by directly binding to BDNF receptor TrkB. Nat Neurosci. 2023;26:1032–41.
Buckley PF, Mahadik S, Pillai A, Terry A Jr. Neurotrophins and schizophrenia. Schizophr Res. 2007;94:1–11.
Calder AE, Hasler G. Towards an understanding of psychedelic-induced neuroplasticity. Neuropsychopharmacology. 2023;48:104–12.
Martin DA, Marona-Lewicka D, Nichols DE, Nichols CD. Chronic LSD alters gene expression profiles in the mPFC relevant to schizophrenia. Neuropharmacology. 2014;83:1–8.
Nichols CD, Sanders-Bush E. A single dose of lysergic acid diethylamide influences gene expression patterns within the mammalian brain. Neuropsychopharmacology. 2002;26:634–42.
Yaden DB, Griffiths RR. The subjective effects of psychedelics are necessary for their enduring therapeutic effects. ACS Pharmacol Transl Sci. 2021;4:568–72.
Higgins GA, Carroll NK, Brown M, MacMillan C, Silenieks LB, Thevarkunnel S, et al. Low doses of psilocybin and ketamine enhance motivation and attention in poor performing rats: evidence for an antidepressant property. Front Pharmacol. 2021;12: 640241.
Rootman JM, Kryskow P, Harvey K, Stamets P, Santos-Brault E, Kuypers KPC, et al. Adults who microdose psychedelics report health related motivations and lower levels of anxiety and depression compared to non-microdosers. Sci Rep. 2021;11:22479.
Liechti ME, Holze F. Dosing psychedelics and MDMA. Curr Top Behav Neurosci. 2022;56:3–21.
Gonzalez-Maeso J, Sealfon SC. Agonist-trafficking and hallucinogens. Curr Med Chem. 2009;16:1017–27.
Cameron LP, Tombari RJ, Lu J, Pell AJ, Hurley ZQ, Ehinger Y, et al. A non-hallucinogenic psychedelic analogue with therapeutic potential. Nature. 2021;589:474–9.
Cao D, Yu J, Wang H, Luo Z, Liu X, He L, et al. Structure-based discovery of nonhallucinogenic psychedelic analogs. Science. 2022;375:403–11.
Olson DE. Psychoplastogens: a promising class of plasticity-promoting neurotherapeutics. J Exp Neurosci. 2018;12:1179069518800508.
Funding
This work was supported by the statutory activity of Maj Institute of Pharmacology, Polish Academy of Sciences, Kraków, Poland.
Author information
Authors and Affiliations
Contributions
MM: conception of the work and writing of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Maćkowiak, M. Psychedelics action and schizophrenia. Pharmacol. Rep 75, 1350–1361 (2023). https://doi.org/10.1007/s43440-023-00546-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43440-023-00546-5