Abstract
Maternal subclinical hypothyroidism (SCH) during pregnancy can adversely affect the neurodevelopment of the offspring. The balance of nerve growth factor (NGF)-related tropomyosin receptor kinase A/p75 neurotrophin receptor (TrkA/p75NTR) signaling in the hippocampus is important in brain development, and whether it affects cognitive function in maternal SCH’s offspring is not clear. In this study, we found that compared with the control (CON) group, expression of proliferation-related proteins [NGF, p-TrkA, phospho-extracellular signal-regulated kinase 1/2 (p-ERK1/2) and phospho-cAMP response element-binding protein (p-CREB)] decreased in the hippocampus of the offspring in the SCH group, overt hypothyroidism (OHT) group, and the group with levothyroxine (L-T4) treatment for SCH from gestational day 17 (E17). In contrast, expression of apoptosis-related proteins [pro-NGF, p75NTR, phospho-C-Jun N-terminal kinase (p-JNK), p53, Bax and cleaved caspase-3] was increased. The two groups with treatment with L-T4 for SCH from E10 and E13, respectively, showed no significant difference compared with the CON group. L-T4 treatment enhanced relative expression of NGF by increasing NGF/proNGF ratio in offspring from maternal SCH rats. In conclusion, L-T4 treatment for SCH from early pregnancy dramatically ameliorated cognitive impairment via TrkA/p75NTR signaling, which involved activation of the neuronal proliferation and inhibition of neuronal apoptosis in SCH rats’ offspring.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Thyroid hormones (THs) are essential for brain development and have a critical role in fetal brain development [1, 2]. TH deficiency during development can cause obvious and irreversible damage to neurodevelopment unless hormone replacement therapy is initiated on time [3]. Subclinical hypothyroidism (SCH) is the most common form of thyroid dysfunction in pregnancy with a prevalence of 3–5% [4, 5]. In the past few decades, epidemiological investigations have revealed that maternal SCH during pregnancy may have an impact on brain development in the offspring [6,7,8]. In the light of this phenomenon, our group found that the neurodevelopment of offspring that were born from rats with SCH during pregnancy was significantly worse than that from normal control rats, based on results from Morris water-maze (MWM) and long-term potentiation (LTP) [9,10,11,12,13]. We have also reported that the expression of brain-derived neurotrophic factor (BDNF) and its downstream molecules are altered in the offspring of maternal rats with SCH [11, 12]. Nerve growth factor (NGF) and BDNF are classical neurotrophic factors that regulate cell differentiation, proliferation, and survival; promote axon and dendritic growth; enhance synaptic plasticity; and inhibit apoptosis [14, 15]. NGF mainly binds to tropomyosin receptor kinase (Trk)A, which activates cAMP response element-binding protein (CREB) to regulate regeneration, survival, and proliferation of neurons [16]. Meanwhile, the p75 neurotrophin receptor (p75NTR) is a low-affinity receptor for NGF and promotes neuronal apoptosis by activating the C-Jun N-terminal kinase (JNK) signaling pathway [17, 18]. NGF plays a critical role in neurodevelopment by activating the TrkA signaling pathway and inhibiting the p75NTR signaling pathway [14, 19,20,21,22]. Reduction of NGF in the cerebral cortex and cerebellum has been detected in the offspring of rats with perinatal hypothyroidism. This leads to inhibition of the TrkA signaling pathway, activation of the p75NTR signaling pathway, and imbalance in the TrkA/p75NTR signaling pathway [23, 24]. However, whether an imbalance of the TrkA/p75NTR signaling pathway is found in hippocampal development in offspring of SCH rats has not been clarified.
The present study was designed to determine whether the TrkA/p75NTR signaling pathway was imbalanced in the hippocampus of the offspring of maternal SCH rats, and to study the effects on the brain development of levothyroxine (L-T4) replacement therapy started at different times during pregnancy. Finally, we offer a theoretical basis for clinicians to choose the optimal intervention time for SCH women during pregnancy.
Materials and methods
Animals
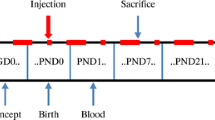
Ninety nulliparous female Wistar rats (weighing 190–210 g) were housed in cages in a climate-controlled, specific-pathogen-free environment (23–25 °C, 55% relative humidity, 12-h light/dark photoperiod) with free access to normal rat chow and tap water in the Experimental Animal Department of China Medical University. All animals and experimental procedures were approved by the Animal Care and Use Committee of China Medical University, which complied with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publications No. 8023, revised 1978). After a 1-week adaptation period, we randomly divided all rats into two groups: 15 of 90 underwent sham thyroid surgery as the control (CON) group, and others underwent thyroidectomy, all operation were done after intraperitoneal (i.p.) injection of 3% pentobarbital sodium (0.1 mL/100 g). Calcium lactate (0.1% w/v) was added to the drinking water for all rats after surgery. One month later, 15 of 75 rats after thyroidectomy were randomly selected as the overt hypothyroidism (OHT) group. The remaining 60 rats were used to establish SCH models by subcutaneous (s.c.) injection of L-T4 (Sigma-Aldrich, St. Louis, MO, USA) in the neck at a concentration of 1.0 μg/100 g/day. Rats in the CON and OHT groups received s.c. injection of physiological saline (50 µL/100 g/day) in the same position. After 9 days, all 90 female rats copulated with normal male rats (male: female = 1: 2). The next morning, if sperm was found in vaginal smears, the day was set as day 0 of pregnancy (E0). The 60 thyroidectomized rats that received L-T4 injections were further randomly divided into four groups of 15: the SCH group continued L-T4 injections (1.0 μg/100 g/day), and other rats received an L-T4 replacement treatment (1.25 µg/100 g/day) to maintain normal thyroid hormone levels starting from E10, E13, and E17, respectively. The female rats were observed daily until they gave birth, and the delivery day was recorded as postnatal day 0 (PND0). All offspring weaned from PND21 and the female rats stopped subcutaneous injection meanwhile.
Blood samples (approximately 2 mL) were collected from the orbital vein of all pregnant rats 9 days after injection and PND0 to test the level of total thyroxine (TT4) and thyroid-stimulating hormone (TSH). On PND7, the pups were decapitated on ice for western blotting (N = 6), immunofluorescence and terminal deoxynucleotidyl transferase-mediated dUTP nick end-labeling (TUNEL) staining (N = 6). The remaining pups were raised to PND39 for MWM tests (n = 10) and LTP induction assay (n = 6). The project timeline is described in Fig. 1.
Measurements of TT4 and TSH
Blood samples obtained from rats were centrifuged at 20,913 g for 15 min immediately and stored at − 80 °C for subsequent measurement including TT4 and TSH. TT4 was calculated by Roche ElectroChemiLuminescence (Roche Diagnostic Products, Los Angeles, CA, USA). TSH was measured by ELISA Kit for Thyroid Stimulating Hormone (Cloud-Clone Corp., Houston, TX, USA). The inter- and intra-assay coefficients of variation (CVs) for TT4 were 3.3–4.2 and 1.3–1.8%, respectively. The inter- and intra-assay CVs for TSH were < 10 and < 12%, respectively. All samples for TT4 and TSH measurements were performed in duplicate.
MWM test
The MWM test (including navigation tests and a probe trial test) was utilized to appraise the degree of spatial learning in pups from all groups. The MWM consisted of a black circular swimming pool (100 cm diameter × 60 cm depth) divided into four equal quadrants (I–IV) by two straight lines. A circular platform (10 cm diameter) was placed in the middle of quadrant IV and was sunk 1 cm below the surface of the water. Starting from PND39, the pups were randomly selected from each group to finalize the test (N = 10). On the first day, the pups were allowed to swim freely for 120 s to adapt to the water maze and platform. On the second day (PND40), the pups started training; each pup was trained four times from the four different quadrants per day. The time of pups finding the platform was defined as escape latency. Once the pup climbed on the platform, escape latency was recorded and the pup was recycled gently after staying on the platform for 10 s. If a pup failed to find the platform after 120 s, it was guided to the platform and the escape latency was registered as 120 s. We repeated the aforementioned training until the fifth day (PND40, PND41, PND42, and PND43). On the sixth day, the probe trial was performed to assess long-term memory. The platform was removed and the experiments performed on the previous 4 days were repeated. We recorded the number of times each pup reached the platform area and the time spent in the area, which was assigned as the probe trial.
LTP induction
Synaptic plasticity in the CA1 area of the hippocampus at 6 weeks can be evaluated by high-frequency stimulation (HFS)-induced increase in field-excitatory postsynaptic potentials (f-EPSPs). We measured f-EPSPs in the hippocampus of the pups from each group using a MED64 planar microelectrode matrix recording system (Alpha Med Science, Osaka, Japan). Pups were anesthetized with 3% sodium pentobarbital (30 mg/kg, i.p.) on PND40 (N = 6). The brains were immediately removed and cut into 300-μm-thick slices. Brain slices were incubated in artificial cerebrospinal fluid (ACSF): 14.5 g NaCl, 0.44 g KCl, 4.36 g NaHCO3, 0.24 g MgSO4, 0.34 g KH2PO4, 3.6 g D-glucose, and 0.44 g CaCl2 in 2 L deionized water (pH 7.35–7.45). The temperature of ACSF was maintained at 33–35 °C and the flow rate at 1–1.5 mL/min. Simultaneously, the mixed gas containing 95% O2 and 5% CO2 passed to maintain these brain slices. The baseline level before HFS was measured and stabilized for 10–15 min. Input/output curves were obtained by increasing the intensity of the stimulus and adjusting it to elicit 70% of the maximum response. The f-EPSP was recorded and the stimulus value corresponding to a 50% amplitude difference was recorded as the HFS. The tissue was stimulated twice with HFS, and LTP was induced after 10 s and recorded for > 30 min. The f-EPSP% increase after HFS was used as an indicator for evaluating LTP.
Western blotting
On PND7, the pups were sacrificed on ice after anesthesia (3% sodium pentobarbital, 0.1 mL/100 g, i.p.) (N = 6). The hippocampus was removed immediately and every 0.1 g hippocampus tissue was added to 500 μL buffer containing protease phosphatase inhibitors and phenylmethanesulfonylfluoride (Keygen Biotech, Nanjing, China). The tissue was homogenized by shaking and centrifugation at 20,931 g for 15 min at 4 °C. Protein concentrations were determined by the bicinchoninic acid method (Beyotime, Shanghai, China), and the samples were stored at − 80 °C. Tissue lysates were diluted to the same protein concentrations (3 μg/μL), boiled for 8 min, and 10-μL (30 g protein) samples from each group were subjected to 10% SDS-PAGE. The markers were separated at a constant 80 V for 30 min, and the proteins were separated at a constant 120 V for 1 h. After electrophoresis, the proteins were transferred to polyvinylidene difluoride membranes (Millipore, Billerica, MA, USA) at a constant of 100 V for 1.5 h. Nonspecific binding was blocked using a mixture of Tris-buffered saline and 0.05% Tween-20 with the skimmed milk powder, except for phospho-C-Jun N-terminal kinase (p-JNK), phospho-cAMP response element-binding protein (p-CREB), phospho-extracellular signal-regulated kinase (p-ERK) and phospho-tropomyosin receptor kinase A (p-TrkA) that were blocked with bovine serum albumin (BSA). The membranes were incubated overnight at 4 °C with the following antibodies: rabbit anti-NGF (1:1000 dilution; Abcam, ab52918, Cambridgeshire, UK); rabbit anti-proNGF (1:1000 dilution; Sigma-Aldrich, P5498); rabbit anti-total TrkA (1:1000 dilution; Cell Signaling Technology 2505S, USA); rabbit anti-p-TrkA (1:1000 dilution; Abcam, ab1445); rabbit anti-total ERK (1:1000 dilution; CST4695S); rabbit anti-p-ERK (1:2000 dilution; CST4370S); rabbit anti-total CREB (1:1000 dilution; CST9197S); rabbit anti-p-CREB (1:1000 dilution; CST9198S); rabbit anti-p75NTR (1:1000 dilution; CST8238S); rabbit anti-p53 (1:1000 dilution; CST32532S); rabbit anti-total JNK (1:1000 dilution; CST9252S); rabbit anti-p-JNK (1:1000 dilution; CST4668S); rabbit anti-Bax (1:1000 dilution; CST2772S); and rabbit anti-cleaved caspase-3 (1:1000 dilution; CST9664S). The blots were incubated for 1.5 h with horseradish-peroxidase-conjugated secondary antibody (1:5000 dilution; ZB2301, Beijing, China) and developed by chemiluminescent western blotting (ALPHAVIEW, version 1.3; Protein Simple Inc., San Jose, CA, USA). The optimal time to expose the blot to the membrane was determined by standardization experiments.
Immunofluorescence
On the PND7, pups were transcardially perfused with saline and then with 4% paraformaldehyde solution at pH 7.4 (N = 6). The brains were removed from the skull, fixed with the same fixative for 5 days, and transferred to 10, 20, and 30% sucrose solution for dehydration for 2 days each. After dehydration was completed, the brains were cut into 10-μm-thick pieces using a freezing microtome (− 20 °C, CM1950; Leica, Wetzlar, Germany) on glass slides and stored at − 20 °C. Four random sections were obtained from each pup’s brain. The tissue sections were washed three times in phosphate-buffered saline with 0.05% Tween-20 (PBST) and incubated in a blocking solution containing 5% BSA and 0.3% Triton X-100 at room temperature for 1 h. These tissue sections were incubated overnight with primary antibodies: rabbit anti-p-CREB (1:400 dilution; CST9198S), rabbit anti-cleaved caspase-3 (1:800 dilution; CST9664S) and mouse anti-NeuN (1:500 dilution; NBP1-92,693, Colorado, USA) at 4 °C. On the next day, after PBST washing, the sections were incubated for 2 h with fluorochrome-tagged specific secondary antibodies: Alexa Flour 488-conjugated Affinipure Goat Anti-Rabbit IgG(H + L) (1:300 dilution; SA00013-2, Chicago, Proteintech); CoraLite 594-conjugated Goat Anti-Rabbit IgG(H + L) (1:100 dilution; CL594-66,467, Proteintech); and AMCA-conjugated Affinipure goat anti-mouse IgG(H + L) (1:20 dilution; SA00010-1, Proteintech) at room temperature away from light. Finally, the sections were mounted in an anti-fade mounting medium and examined hippocampus CA1 by confocal microscopy (20X, Leica TCS SP5 II, Germany).
TUNEL staining
On the PND7, TUNEL staining was performed on the frozen tissue sections using the In Situ Cell Death Detection Kit, POD (11,684,817,910, Roche, Sigma, USA). The sections were fixed in freshly prepared 4% paraformaldehyde solution at pH 7.4 for 1 h at 15–25 °C. The sections were blocked in 3% H2O2 for 10 min at 15–25 °C, after rinsing three times with PBS. The sections were permeabilized by freshly prepared 0.1% Triton X-100 in 0.1% sodium citrate for 2 min on ice (2–8 °C), after rinsing three times with PBS. Sections were rinsed twice with PBS and we dried the area around the sample, followed by incubation with TUNEL reaction mixture for 1 h at 37 °C in a humidified atmosphere in the dark. The sections were rinsed three times with PBS and analyzed in a drop of PBS under confocal microscopy to exam hippocampus CA1 (20X, Leica TCS SP5 II), after being counterstained with 1 µg/mL 4′,6-diamidino-2-phenylindole (DAPI) dye.
Statistical analysis
Data processing and statistics were conducted using SPSS 22.0 software (SPSS, Chicago, IL, USA). The results were expressed as the mean ± standard deviation (SD). Multiple group comparisons were performed using ANOVA followed by Dunnett’s T3 test. p < 0.05 was considered to be statistically significant. All artwork was created by GraphPad Prism 7.0 (La Jolla, CA, USA).
Results
TH levels in pregnant rats
We assessed serum TT4 and TSH levels in maternal rats at 9 days after L-T4 injection and PND1 to confirm the established success of the SCH, OHT, and L-T4 treatment models. The results of TSH and TT4 of each group of rats at 9 days of injection and PND1 were shown in Table 1. The OHT group showed a significant increase in TSH and decrease in TT4 compared with the CON group at 9 days after L-T4 injection and PND1. Compared with the CON group, TSH levels were significantly higher in SCH rats. Nevertheless, there was no significant difference in TT4 levels at 9 days after L-T4 injection and PND1. SCH pregnant rats’ serum TT4 levels showed significantly higher than the OHT group. Before L-T4 treatment, rats in the three treatment groups had a similar thyroid status compared with the SCH group. After L-T4 treatment at 1.25 μg/100 g/day, TSH levels of maternal rats in three treatment groups were suppressed significantly (p < 0.05) compared with the SCH group. No significant difference was observed in serum TT4 level between the CON and L-T4 treatment groups at 9 days after L-T4 injection and PND1. These results indicated that treatment with L-T4 was successful and additional L-T4 supplements recovered normal thyroid status in maternal rats. The SCH, OHT, and L-T4 treatment group models were established successfully throughout the pregnancy (Fig. 2).
Thyroid-stimulating hormone (TSH) (a), and serum total thyroxine (TT4) (b) levels in pregnant rats (9 days of injection, N = 12; PND1, N = 12). *p < 0.05 versus same day CON group; #p < 0.05 versus same day SCH group; &p < 0.05 versus same day OHT group. CON, control; PND1, postnatal day 1; OHT, overt hypothyroidism; SCH, subclinical hypothyroidism; TSH, thyrotropin; TT4, total thyroxine
The adverse effect of maternal SCH on spatial learning and memory in offspring
MWM was used to evaluate spatial learning and memory. In all the offspring, escape latency decreased with increased training. Escape latency from PND40 to PND43 was significantly longer in the SCH group compared with the CON group, and that in the OHT group was the longest (p < 0.05). Compared with the SCH group, the latency of the three L-T4 treatment groups was shortened. Although there were significant differences between the E10 and E13 L-T4 treatment groups and the CON group on PND40 (p < 0.05), no significant differences on PND 41–43 (p > 0.05), and no significant difference between the E17 L-T4 treatment and SCH groups (p > 0.05). Starting from training, there was no significant difference in the escape latencies between the CON group and the E10 and E13 L-T4 treatment groups (p > 0.05). On the last day of training, there was no significant difference between the three L-T4 treatment groups and the CON group (Fig. 3a).
Performance of pups in the MWM test (N = 10, PND39-43) and LTP (N = 6, PND40). Data are expressed as the mean ± SD. a. Average time to find the hidden platform was longer in the SCH and OHT groups compared with the CON group from PND 40 to 43. b. Probe trial test recorded the number of times pups crossed the platform quadrant. c. Maternal SCH caused LTP damage in the hippocampal CA1 region in their pups. LTP was induced by HFS and measured as an increase in f-EPSP slope, expressed as a percentage of the baseline of the f-EPSP slope after HFS in all groups. f-EPSP slopes were reduced in the SCH and OHT groups compared with the CON group (p < 0.05). d. Intuitively represent the f-EPSP slope after HFS (% of baseline). *p < 0.05 versus same day CON group; #p < 0.05 versus same day SCH group; &p < 0.05 versus same day OHT group. CON, control; E, gestational day; f-EPSP, field excitatory postsynaptic potential; HFS, high-frequency stimulation; LTP, long-term potentiation; MWM, Morris water maze; OHT, overt hypothyroidism; PND, postnatal day; SCH, subclinical hypothyroidism
There was no significant difference in the number of times that the pups crossed the platform quadrant in the probe trial on PND44 between the SCH and E17 L-T4 treatment groups (p > 0.05). However, they were markedly lower than in the CON, E10, and E13 groups, but significantly higher than in the OHT group (p < 0.05) (Fig. 3b). These results showed that spatial learning and memory were impaired in the offspring from SCH rats. L-T4 treatment starting from E10 and E13 ameliorated the adverse effect of maternal SCH on spatial learning and memory in offspring.
Decrease of LTP in offspring from maternal SCH rats
Spatial learning and memory require synaptic transmission, which is characterized as the LTP of f-EPSPs. The LTP results were evaluated by measuring baseline f-EPSP% after HFS. There were no significant differences in f-EPSP among all groups before HFS (p > 0.05), and all six groups showed a significant increase after HFS. The amplification percentage of the f-EPSP slope in the SCH and OHT groups was significantly lower than that in the CON group (p < 0.05). L-T4 treatment ameliorated the amplification percentage of the f-EPSP slope in the E10 and E13 groups compared with the CON group, and it was significantly higher than that of the SCH and OHT groups (p < 0.05). L-T4 treatment starting from E17 also increased the amplification percentage of the slope of the f-EPSPs compared with the percentage in the SCH group, but the difference was not significant. The slope of the f-EPSPs was still lower than that of the CON group (p < 0.05). Pups in the OHT group demonstrated the lowest levels of amplification percentage of the slope of the f-EPSPs (Fig. 3c–d). These results suggested that SCH during pregnancy damaged LTP in the offspring. L-T4 treatment on E10 and E13 could have prevented LTP decrease in SCH rat offspring.
Expression of NGF, proNGF and calculated the NGF/proNGF ratio in offspring from SCH and L-T4 treatment groups
We measured the expression of NGF and proNGF and NGF/proNGF ratio in the hippocampus from all six groups. Western blotting showed that expression of proNGF increased in SCH (1.58-fold) and OHT (1.78-fold) groups’ offspring compared with the CON group (both p < 0.05). Compared with the SCH group, expression of proNGF was restored in the offspring of the E10 groups (p < 0.05). And L-T4 treatment in the three groups restored proNGF to levels that were not significantly different from those in the CON group (all p > 0.05). In the E17, SCH and OHT groups, NGF levels were significantly reduced to 65, 33 and 26% of the control levels, respectively (all p < 0.05). Compared with the SCH group, expression of NGF was restored to some extent in the rats treated with L-T4 from E10, E13, and E17 (all p < 0.05; Fig. 4a–c). NGF/proNGF ratio in the SCH and OHT groups were significantly lower than in the CON group (both p < 0.05; Fig. 4c), and the OHT group had the largest decline. In the E10 group, NGF/proNGF ratio returned to a level that was not significantly different from that in the CON group (p > 0.05; Fig. 4c). NGF/proNGF ratio in the E13 and E17 groups was still significantly lower than that in the CON group (p < 0.05; Fig. 4c). These results suggested that SCH during pregnancy could have unbalanced TrkA/p75NTR in the hippocampus of offspring. Timely L-T4 treatment during pregnancy ameliorated the balance of TrkA/p75NTR by augmenting the NGF/proNGF ratio.
Expression of NGF (a, c) and its precursor neurotrophin pro-NGF (a, b), as well as the ratio of NGF and pro-NGF (d) in the hippocampus of pups (N = 6, PND7). *p < 0.05 versus CON group, #p < 0.05 versus SCH group, &p < 0.05 versus OHT group. CON, control; E, gestational day; NGF, nerve growth factor; OHT, overt hypothyroidism; SCH, subclinical hypothyroidism
Expression of proliferation-associated NGF/TrkA pathway in offspring from SCH and L-T4 treatment groups
We investigated the NGF/TrkA-related signaling pathway and related substrates downstream in offspring of SCH and L-T4 treatment groups. Western blotting showed that compared with the CON group, expression of p-TrkA (Fig. 5a, b), p-ERK1/2 (Fig. 5c, d), and p-CREB (Fig. 5e, f) were decreased in the hippocampus of offspring from SCH and OHT rats (all p < 0.05), but the total protein levels had no significant differences, respectively (all p > 0.05, Fig. 5c, f, i). Compared with the SCH group, the expression of p-TrkA and p-CREB increased significantly in L-T4 treatment from E10 and E13 (p < 0.05), and three L-T4 treatment groups p-ERK1/2 were increased significantly (p < 0.05). Nevertheless, the total protein levels had no significant differences among all six groups (p > 0.05).
Expression of phospho-tropomyosin-related kinase A (p-TrkA) (a, b), phospho-extracellular signal-regulated kinase 1/2 (p-ERK1/2) (c, d) and phospho-cAMP response element-binding protein (p-CREB) (e, f) in the hippocampus of pups (N = 6, PND7). *p < 0.05 versus CON group, #p < 0.05 versus SCH group, &p < 0.05 versus OHT group. CON, control; E, gestational day; OHT, overt hypothyroidism; SCH, subclinical hypothyroidism
Immunofluorescence showed that expression of p-CREB was consistent with that in western blotting (Fig. 7a, d). These results indicated that SCH during pregnancy reduced expression of proliferation-associated NGF/TrkA pathway in the hippocampus of offspring and decreased neuronal proliferation. Treatment with L-T4 treatment from E10 and E13 reversed the decrease in NGF/TrkA pathway in offspring of SCH rats.
Expression of apoptosis-associated proNGF/p75NTR pathway in offspring from SCH and L-T4 treatment groups
We investigated the proNGF/p75NTR-related signaling pathway and related substrates downstream in offspring of SCH and L-T4 treatment groups. Western blotting showed that, compared with the CON group, p75NTR (Fig. 6a), Bax (Fig. 6b), p53 (Fig. 6c), cleaved caspase-3 (Fig. 6d), and p-JNK (Fig. 6e) were overexpressed in pups from the SCH and OHT groups. In the hippocampus of offspring from the three L-T4 treatment groups (E10, E13, and E17), protein expression was increased to varying degrees. However, the total JNK levels were similar among all groups (Fig. 6i and k).
Expression of p75 neurotrophin receptor (p75NTR) (a), Bax (b), proapoptotic proteins p53 (c) and cleaved caspase-3 (d), and phospho-c-Jun N-terminal kinase (p-JNK) (e) in the hippocampus of pups (N = 6, PND7). *p < 0.05 versus CON group, #p < 0.05 versus SCH group; &p < 0.05 versus OHT group. CON, control; E, gestational day; OHT, overt hypothyroidism; SCH, subclinical hypothyroidism
The SCH group showed less expression of cleaved caspase-3 than the OHT group but higher expression than the CON and L-T4 treatment groups (Fig. 7b, e). Consistent with the cleaved caspase-3 results, the number of TUNEL-positive cells in the hippocampus was significantly increased in the SCH and OHT groups compared with the CON group. Offspring in the L-T4 treatment groups showed fewer TUNEL-positive cells in the hippocampus than those in the SCH and OHT groups (Fig. 7c, f). These results indicated that SCH during pregnancy activated expression of the apoptosis-associated NGF/75NTR pathway in offspring to increase neuronal apoptosis. L-T4 treatment on E10 and E13 reversed the increase in the NGF/p75NTR pathway in offspring of SCH rats.
The results of immunofluorescence and TUNEL staining in the hippocampus of pups (N = 6, PND7). a. Double immunofluorescence with antibodies against NeuN (green) and phospho-cAMP response element-binding protein (p-CREB) (red) demonstrated the expression of p-CREB on hippocampal neurons. b. Double immunofluorescence with antibodies against NeuN (green) and cleaved caspase-3 (blue) demonstrated the expression of cleaved caspase-3 on hippocampal neurons. c. The neural apoptosis in the hippocampus, as shown by TUNEL assay (TUNEL, green; DAPI, blue). The merged images showed overlapping localization. d–f. Quantitative analysis of the relative expression level expression of p-CREB, cleaved caspase-3 and TUNEL+. Scale bar = 500 μm. TUNEL, terminal deoxynucleotidyl transferase-mediated dUTP nick end-labeling
Discussion
Previous studies have demonstrated that maternal SCH can adversely affect the neurodevelopment of offspring, both in humans [6, 8] and animals [9,10,11,12,13]. Our group has found that many neurodevelopment-related proteins in the progeny of maternal SCH rats are reduced, such as BDNF, p-ERK, p-CREB, p-CaMKIV, p75NTR [11,12,13]. This study continued to explore the mechanism of impaired neurocognitive ability caused by maternal SCH that imbalance of NGF-related TrkA/p75NTR signaling in the hippocampus of offspring in SCH rats.
There are two main stages in the prenatal development of mammals in terms of TH synthesis and release into the fetal nervous system. In stage I, the first trimester of pregnancy, fetal brain development requires a large number of THs from the maternal coelomic and/or amniotic fluid [25]. In stage II, the fetal thyroid begins to develop, and the THs from the fetus and mother work together [25, 26]. At birth, the brain development of the fetus is dependent entirely on the THs secreted by the neonatal thyroid [27]. We selected gestational time points in rats that corresponded to those in humans. The fetal thyroid does not begin to develop until E11 and serves a useful function by E17 [28]. Therefore, we chose E10 of rats as equivalent to week 8 of a human pregnancy, E13 as week 12 and E17 as week 16. We used thyroidectomy combined with supplementation with L-T4 to establish the SCH and L-T4 treatment group models. The dose of L-T4 was based on the results of serum thyroid function indexes, which is consistent with previous studies [11].
Compared with the CON group, spatial learning and memory in the SCH group pups were significantly decreased when evaluated by the MWM test. These impairments were more severe in the OHT group. Additionally, L-T4 treatment from E10 and E13 improved the impairments close to the levels of the CON group. These results are in agreement with the study of Wang et al. [11]. Our results also showed that more training sessions had a greater effect on escape latencies. The probe trial test showed that, compared with the CON group, SCH and OHT groups’ pups had impairment of sustained memory, which was restored by L-T4 treatment from E10 and E13. These results suggested that the progeny of maternal SCH and OHT rats may have irreversible damage to neurodevelopment, and the neural network connectivity cannot be restored even after prolonged training.
Another method to assess the ability of learning and memory is measuring synaptic plasticity by LTP in the hippocampus of pups. The amplitude and f-EPSP slope in the CON group were significantly higher than those in the SCH and OHT groups, which indicated damaged LTP. LTP in the E10 and E13 L-T4 treatment groups showed that f-EPSP increased significantly compared with the SCH group. This demonstrated that L-T4 treatment from E13 at least could moderate the effect of SCH on offspring’s synaptic plasticity, which was in line with previous studies [11].
TH deficiency can result in the reduction of the number of neurons, affect the course of neuronal proliferation and migration, reduce synaptic plasticity, and increase neuronal apoptosis in rodents. These factors may be the potential mechanism by which SCH impairs spatial learning and memory [13, 29, 30]. These physiological processes are further regulated by NGF [31,32,33,34]. It is known that NGF can activate the NGF/TrkA signaling pathway and function in neuronal proliferation and migration as well as synaptic plasticity. The NGF/p75NTR signaling pathway can be suppressed by TrkA activation to elicit antiapoptotic effects. In contrast, as the precursor protein of NGF, proNGF can bind to p75NTR easier than NGF and elicit neuronal apoptosis [35,36,37,38]. ProNGF can switch between neurotrophic and apoptotic activity by responding to changes in TrkA receptor levels, whereas mature NGF cannot [39]. Casponi et al. found that, as NGF was neutralized by the recombinant antibody in AD11 anti-NGF transgenic mice, proNGF was predominant, which led to p75NTR-dependent apoptosis and cognitive deficits. The p75NTR-dependent apoptosis and cognitive defect could be fully reversed by NGF supplementation [37]. Therefore, the relative expression level of NGF and proNGF (NGF/proNGF ratio) plays an important role in maintaining the TrkA/p75NTR balance [40]. Once TrkA/p75NTR are out of balance, apoptosis is higher than the proliferation of neurons, which is not conducive to the development of the nervous system, and results in the suboptimal neurocognitive ability of the offspring. Our results showed that due to the lack of NGF, the ratio of NGF/proNGF was imbalanced, which prompted an imbalance of the TrkA/p75NTR pathways. In turn, it disrupted the balance of neuronal proliferation and apoptosis, and impaired the development of memory and learning ability of the offspring from maternal SCH. Moreover, our results showed that timely supplementation with L-T4 in SCH rats can activate the neuroprotective effects by adjusting the TrkA/p75NTR imbalance status in pups’ neurodevelopment.
NGF can activate TrkA receptors and trigger three main signaling pathways: Ras/ERK, PLC-α, and PI3-kinase/Akt [41], then complete physiological activities, including neuronal survival, outgrowth and differentiation. ERK1/2 is the key to conducting signals from surface receptors to the nucleus. Active phosphorylation of ERK1/2 is involved in the proliferation, differentiation and apoptosis of neurons [42]. p-ERK1/2 can be translocated to the nucleus, where CREB is phosphorylated after interacting with other molecules [43, 44]. As an important molecule for amyloidosis, learning and memory, CREB plays a crucial role in neurodevelopment. Hence, we also detected the expression of p-CREB by immunofluorescence for further confirmation. In our experiments, TH deficiency decreased the amount of NGF, inhibited activity of TrkA, and decreased expression of p-ERK and p-CREB, which can be restored by timely supplementation with L-T4 to normal thyroid function during early pregnancy (Fig. 8).
Effects of SCH and OHT on NGF-mediated TrkA and p75NTR signaling pathway. NGF induces TrkA signaling pathway to increase neuronal proliferation. Pro-NGF, the precursor protein of NGF, can combine with p75NTR preferentially to enhance neuronal apoptosis. CREB, cAMP response element-binding protein; JNK, C-Jun N-terminal kinase; NGF, nerve growth factor; OHT, overt hypothyroidism; p75NTR, p75 neurotrophin receptor; SCH, subclinical hypothyroidism; TrkA, tropomyosin-related kinase A
The p75NTR is a low-affinity receptor for NGF and binds with cell ligands to play a dual (proliferative/apoptotic) role in neurogenesis [45], and the JNK signaling pathway is a key step in this process [46]. p53, a genome guardian, is actuated by diverse stresses and stimuli and mediates DNA repair, apoptosis, and cell cycle arrest [47]. BAX, a key member of the Bcl-2 family of proteins, mediates apoptosis and releases cytochrome C from the mitochondrial membrane, through possible mechanisms that include selective disruption of the outer membrane as a result of mitochondrial matrix hyperpolarization/matrix swelling [48,49,50]. Apoptosomes are formed by cytochrome c with apoptotic protease activating factor-1(Apaf-1) and caspase-9 activates caspase-3 downstream [51]. As caspase-3 is a typical marker of apoptosis, we also detected its expression levels by immunofluorescence for further confirmation. In our experiments, TH deficiency increased the cleavage activity of p75NTR, activated JNK signaling, enhanced expression of p53, BAX and cleaved caspase-3, and increased neuronal apoptosis. These changes are reversed to normal thyroid function by timely supplementation with L-T4 in early pregnancy (Fig. 8).
Conclusion
In conclusion, our study demonstrated that maternal SCH inhibits the TrkA signaling pathway, activated the p75NTR signaling pathway, and unbalanced the TrkA/p75NTR signaling pathway in the hippocampus of pups. This led to the decreased neuronal proliferation and increased neuronal apoptosis in offspring’s hippocampus, causing suboptimal neurocognitive ability. L-T4 treatment from early pregnancy could restore protein levels of the progeny to approximately normal levels, thereby ameliorating the adverse effects of maternal SCH on spatial learning and memory in offspring. We discovered that the offspring of subclinical hypothyroidism during pregnancy have abnormal neurodevelopment, and conducted preliminary research on the underlying mechanism and proposed some feasible directions for future research, such as how TSH and T4 affect the expression of the TrkA/p75NTR signaling pathway.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request. Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.
References
Korevaar TIM, Tiemeier H, Peeters RP (2018) Clinical associations of maternal thyroid function with foetal brain development: epidemiological interpretation and overview of available evidence. Clin Endocrinol (Oxf) 89(2):129–138
Moog NK, Entringer S, Heim C, Wadhwa PD, Kathmann N, Buss C (2017) Influence of maternal thyroid hormones during gestation on fetal brain development. Neuroscience 342:68–100
Morreale de Escobar G, Obregon MJ, Escobar del Rey F (2000) Is neuropsychological development related to maternal hypothyroidism or to maternal hypothyroxinemia? J Clin Endocrinol Metab 85(11):3975–3987
Shan ZY, Chen YY, Teng WP, Yu XH, Li CY, Zhou WW et al (2009) A study for maternal thyroid hormone deficiency during the first half of pregnancy in China. Eur J Clin Invest 39(1):37–42
Teng W, Shan Z, Patil-Sisodia K, Cooper DS (2013) Hypothyroidism in pregnancy. Lancet Diabetes Endocrinol 1(3):228–237
Haddow JE, Palomaki GE, Allan WC, Williams JR, Knight GJ, Gagnon J et al (1999) Maternal thyroid deficiency during pregnancy and subsequent neuropsychological development of the child. N Engl J Med 341(8):549–555
Smit BJ, Kok JH, Vulsma T, Briet JM, Boer K, Wiersinga WM (2000) Neurologic development of the newborn and young child in relation to maternal thyroid function. Acta Paediatr 89(3):291–295
Li Y, Shan Z, Teng W, Yu X, Li Y, Fan C et al (2010) Abnormalities of maternal thyroid function during pregnancy affect neuropsychological development of their children at 25–30 months. Clin Endocrinol (Oxf) 72(6):825–829
Liu D, Teng W, Shan Z, Yu X, Gao Y, Wang S et al (2010) The effect of maternal subclinical hypothyroidism during pregnancy on brain development in rat offspring. Thyroid 20(8):909–915
Lu L, Yu X, Teng W, Shan Z (2012) Treatment with levothyroxine in pregnant rats with subclinical hypothyroidism improves cell migration in the developing brain of the progeny. J Endocrinol Invest 35(5):490–496
Wang S, Teng W, Gao Y, Fan C, Zhang H, Shan Z (2012) Early levothyroxine treatment on maternal subclinical hypothyroidism improves spatial learning of offspring in rats. J Neuroendocrinol 24(5):841–848
Zhang Y, Fan Y, Yu X, Wang X, Bao S, Li J et al (2015) Maternal subclinical hypothyroidism impairs neurodevelopment in rat offspring by inhibiting the CREB signaling pathway. Mol Neurobiol 52(1):432–441
Zhang F, Chen J, Lin X, Peng S, Yu X, Shan Z et al (2018) Subclinical hypothyroidism in pregnant rats impaired learning and memory of their offspring by promoting the p75(NTR) signal pathway. Endocr Connect 7(5):688–697
Colafrancesco V, Villoslada P (2011) Targeting NGF pathway for developing neuroprotective therapies for multiple sclerosis and other neurological diseases. Arch Ital Biol 149(2):183–192
Lu B, Pang PT, Woo NH (2005) The yin and yang of neurotrophin action. Nat Rev Neurosci 6(8):603–614
Lu J, Wu DM, Hu B, Zheng YL, Zhang ZF, Wang YJ (2010) NGF-Dependent activation of TrkA pathway: a mechanism for the neuroprotective effect of troxerutin in D-galactose-treated mice. Brain Pathol 20(5):952–965
Hempstead BL (2002) The many faces of p75NTR. Curr Opin Neurobiol 12(3):260–267
Roux PP, Barker PA (2002) Neurotrophin signaling through the p75 neurotrophin receptor. Prog Neurobiol 67(3):203–233
Counts SE, Mufson EJ (2005) The role of nerve growth factor receptors in cholinergic basal forebrain degeneration in prodromal Alzheimer disease. J Neuropathol Exp Neurol 64(4):263–272
Copray S, Kust B, Emmer B, Lin MY, Liem R, Amor S et al (2004) Deficient p75 low-affinity neurotrophin receptor expression exacerbates experimental allergic encephalomyelitis in C57/BL6 mice. J Neuroimmunol 148(1–2):41–53
Costantini C, Rossi F, Formaggio E, Bernardoni R, Cecconi D, Della-Bianca V (2005) Characterization of the signaling pathway downstream p75 neurotrophin receptor involved in beta-amyloid peptide-dependent cell death. J Mol Neurosci 25(2):141–156
Huang EJ, Reichardt LF (2003) Trk receptors: roles in neuronal signal transduction. Annu Rev Biochem 72:609–642
Kumar A, Sinha RA, Tiwari M, Pal L, Shrivastava A, Singh R et al (2006) Increased pro-nerve growth factor and p75 neurotrophin receptor levels in developing hypothyroid rat cerebral cortex are associated with enhanced apoptosis. Endocrinology 147(10):4893–4903
Sinha RA, Pathak A, Kumar A, Tiwari M, Shrivastava A, Godbole MM (2009) Enhanced neuronal loss under perinatal hypothyroidism involves impaired neurotrophic signaling and increased proteolysis of p75(NTR). Mol Cell Neurosci 40(3):354–364
Calvo RM, Jauniaux E, Gulbis B, Asuncion M, Gervy C, Contempre B et al (2002) Fetal tissues are exposed to biologically relevant free thyroxine concentrations during early phases of development. J Clin Endocrinol Metab 87(4):1768–1777
Obregon MJ, Calvo RM, Escobar Del Rey F, Morreale de Escobar G (2007) Ontogenesis of thyroid function and interactions with maternal function. Endocr Dev 10:86–98
Porterfield SP, Hendrich CE (1993) The role of thyroid hormones in prenatal and neonatal neurological development—current perspectives. Endocr Rev 14(1):94–106
Barez-Lopez S, Guadano-Ferraz A (2017) Thyroid hormone availability and action during brain development in rodents. Front Cell Neurosci 11:240
Madeira MD, Paula-Barbosa M, Cadete-Leite A, Tavares MA (1988) Unbiased estimate of hippocampal granule cell numbers in hypothyroid and in sex-age-matched control rats. J Hirnforsch 29(6):643–650
Madeira MD, Sousa N, Lima-Andrade MT, Calheiros F, Cadete-Leite A, Paula-Barbosa MM (1992) Selective vulnerability of the hippocampal pyramidal neurons to hypothyroidism in male and female rats. J Comp Neurol 322(4):501–518
Conner JM, Franks KM, Titterness AK, Russell K, Merrill DA, Christie BR et al (2009) NGF is essential for hippocampal plasticity and learning. J Neurosci 29(35):10883–10889
Lagostena L, Rosato-Siri M, D’Onofrio M, Brandi R, Arisi I, Capsoni S et al (2010) In the adult hippocampus, chronic nerve growth factor deprivation shifts GABAergic signaling from the hyperpolarizing to the depolarizing direction. J Neurosci 30(3):885–893
Sola E, Capsoni S, Rosato-Siri M, Cattaneo A, Cherubini E (2006) Failure of nicotine-dependent enhancement of synaptic efficacy at Schaffer-collateral CA1 synapses of AD11 anti-nerve growth factor transgenic mice. Eur J Neurosci 24(5):1252–1264
Lazaridis I, Charalampopoulos I, Alexaki VI, Avlonitis N, Pediaditakis I, Efstathopoulos P et al (2011) Neurosteroid dehydroepiandrosterone interacts with nerve growth factor (NGF) receptors, preventing neuronal apoptosis. PLoS Biol 9(4):e1001051
Song W, Volosin M, Cragnolini AB, Hempstead BL, Friedman WJ (2010) ProNGF induces PTEN via p75NTR to suppress Trk-mediated survival signaling in brain neurons. J Neurosci 30(46):15608–15615
Fortress AM, Buhusi M, Helke KL, Granholm AC (2011) Cholinergic degeneration and alterations in the TrkA and p75NTR balance as a result of pro-NGF injection into aged rats. J Aging Res 2011:460543
Capsoni S, Brandi R, Arisi I, D’Onofrio M, Cattaneo A (2011) A dual mechanism linking NGF/proNGF imbalance and early inflammation to Alzheimer’s disease neurodegeneration in the AD11 anti-NGF mouse model. CNS Neurol Disord Drug Targets 10(5):635–647
Tiveron C, Fasulo L, Capsoni S, Malerba F, Marinelli S, Paoletti F et al (2013) ProNGF\NGF imbalance triggers learning and memory deficits, neurodegeneration and spontaneous epileptic-like discharges in transgenic mice. Cell Death Differ 20(8):1017–1030
Ioannou MS, Fahnestock M (2017) ProNGF, but not NGF, switches from neurotrophic to apoptotic activity in response to reductions in TrkA Receptor Levels. Int J Mol Sci 18(3):599
Liu M, Chen F, Sha L, Wang S, Tao L, Yao L et al (2014) (-)-Epigallocatechin-3-gallate ameliorates learning and memory deficits by adjusting the balance of TrkA/p75NTR signaling in APP/PS1 transgenic mice. Mol Neurobiol 49(3):1350–1363
Niewiadomska G, Mietelska-Porowska A, Mazurkiewicz M (2011) The cholinergic system, nerve growth factor and the cytoskeleton. Behav Brain Res 221(2):515–526
Krawczyk MC, Millan J, Blake MG, Feld M, Boccia MM (2019) Relevance of ERK1/2 post-retrieval participation on memory processes: insights in their particular role on reconsolidation and persistence of memories. Front Mol Neurosci 12:95
Xing J, Ginty DD, Greenberg ME (1996) Coupling of the RAS-MAPK pathway to gene activation by RSK2, a growth factor-regulated CREB kinase. Science 273(5277):959–963
Riccio A, Ahn S, Davenport CM, Blendy JA, Ginty DD (1999) Mediation by a CREB family transcription factor of NGF-dependent survival of sympathetic neurons. Science 286(5448):2358–2361
Barnes M, Eberhart CG, Collins R, Tihan T (2009) Expression of p75NTR in fetal brain and medulloblastomas: evidence of a precursor cell marker and its persistence in neoplasia. J Neurooncol 92(2):193–201
Hempstead B (2002) The many faces of p75NTR. Curr Opin Neurobiol 12(3):260–267
Li Y, Zhang MC, Xu XK, Zhao Y, Mahanand C, Zhu T et al (2019) Functional diversity of p53 in human and wild animals. Front Endocrinol (Lausanne) 10:152
Madesh M, Hajnoczky G (2001) VDAC-dependent permeabilization of the outer mitochondrial membrane by superoxide induces rapid and massive cytochrome c release. J Cell Biol 155(6):1003–1015
Kroemer G, Reed JC (2000) Mitochondrial control of cell death. Nat Med 6(5):513–519
De Giorgi F, Lartigue L, Bauer MK, Schubert A, Grimm S, Hanson GT et al (2002) The permeability transition pore signals apoptosis by directing Bax translocation and multimerization. FASEB J 16(6):607–609
Gupta S, Knowlton AA (2005) HSP60, Bax, apoptosis and the heart. J Cell Mol Med 9(1):51–58
Acknowledgements
This work was supported by The National Natural Science Foundation of China (Grant: 81500605 and 81700697).
Funding
This work was supported by The National Natural Science Foundation of China (Grant: 81500605 and 81700697). The funder had no role in study design, data collection or analysis or in the presentation or publication of the results.
Author information
Authors and Affiliations
Contributions
All the authors materially participated in this work; have read and approved the final manuscript. FZ and XL are the first authors for this study. XY is the corresponding author supervising this work. FZ and XL managed the case and drafted the manuscript. FZ, XL, AL and JC performed analysis on all data interpretation from a literature review. XY, ZS and WT reviewed the manuscript. AL and JC prepared histopathological results. We acknowledge that all authors participated sufficiently in the work and take public responsibility for its content.
Corresponding author
Ethics declarations
Consent to participate and publication
All authors participated in this research, read and approved the final manuscript for publication.
Conflict of interest
The authors declare no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Fan Zhang and Xinyue Lin contributed equally to this work
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhang, F., Lin, X., Liu, A. et al. Maternal Subclinical Hypothyroidism in Rats Impairs Spatial Learning and Memory in Offspring by Disrupting Balance of the TrkA/p75NTR Signal Pathway. Mol Neurobiol 58, 4237–4250 (2021). https://doi.org/10.1007/s12035-021-02403-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-021-02403-z