Abstract
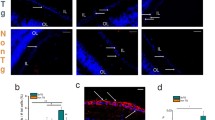
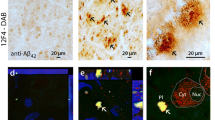
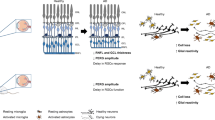
The concept 'the retina as a window to the brain' has been increasingly explored in Alzheimer´s disease (AD) in recent years, since some patients present visual alterations before the first symptoms of dementia. The retina is an extension of the brain and can be assessed by noninvasive methods. However, assessing the retina for AD diagnosis is still a matter of debate. Using the triple transgenic mouse model of AD (3xTg-AD; males), this study was undertaken to investigate whether the retina and brain (hippocampus and cortex) undergo similar molecular and cellular changes during the early stages (4 and 8 months) of the pathology, and if the retina can anticipate the alterations occurring in the brain. We assessed amyloid-beta (Aβ) and hyperphosphorylated tau (p-tau) levels, barrier integrity, cell death, neurotransmitter levels, and glial changes. Overall, the retina, hippocampus, and cortex of 3xTg-AD are not significantly affected at these early stages. However, we detected a few differential changes in the retina and brain regions, and particularly a different profile in microglia branching in the retina and hippocampus, only at 4 months, where the number and length of the processes decreased in the retina and increased in the hippocampus. In summary, at the early stages of pathology, the retina, hippocampus, and cortex are not significantly affected but already present some molecular and cellular alterations. The retina did not mirror the changes detected in the brain, and these observations should be taking into account when using the retina as a potential diagnostic tool for AD.








Similar content being viewed by others
Data Availability
Raw data files are available upon request.
Code Availability
Not applicable.
Abbreviations
- Aβ:
-
Amyloid beta
- AD:
-
Alzheimer´s disease
- AP:
-
Alkaline phosphatase
- APP:
-
Amyloid precursor protein
- BACE:
-
Beta-secretase
- BBB:
-
Blood–brain barrier
- BCA:
-
Bicinchoninic acid
- BRB:
-
Blood–retinal barrier
- BSA:
-
Bovine serum albumin
- CNS:
-
Central nervous system
- ChAT:
-
Choline acetyltransferase
- DAPI:
-
6-Diamidino-2-phenylindole
- DOC:
-
Sodium deoxycholate
- DTT:
-
Dithiothreitol
- ECF:
-
Chemifluorescence
- ECL:
-
Chemiluminescence
- EDTA:
-
Ethylenediaminetetraacetic acid
- EGTA:
-
Ethylene glycol tetraacetic acid
- GABA:
-
Gamma-aminobutyric acid
- GFAP:
-
Glial fibrillary acidic protein
- HBSS:
-
Hank’s balanced salt solution
- HPLC:
-
High-performance liquid chromatography
- HRP:
-
Horseradish peroxidase
- Iba-1:
-
Ionized calcium-binding adapter molecule 1
- PAGE:
-
Polyacrylamide gel electrophoresis
- PBS:
-
Phosphate buffer saline
- PFA:
-
Paraformaldehyde
- PMSF:
-
Phenylmethylsulfonyl fluoride
- PSD95:
-
Postsynaptic density protein 95
- p-tau:
-
Phosphorylated tau
- PVDF:
-
Polyvinylidene difluoride
- RGCs:
-
Retinal ganglion cells
- RT:
-
Room temperature
- SDS:
-
Sodium dodecyl sulfate
- THB:
-
Tissue homogenization buffer
- vGlut1:
-
Vesicular glutamate transporter type I
- WT:
-
Wild-type
- TJ:
-
Tight junctions
- TUNEL:
-
Terminal deoxynucleotidyl transferase dUTP nick end labeling
- ZO-1:
-
Zonula occludens-1
References
London A, Benhar I, Schwartz M (2013) The retina as a window to the brain—from eye research to CNS disorders. Nat Rev Neurol 9(1):44–53. https://doi.org/10.1038/nrneurol.2012.227
Chang LY, Lowe J, Ardiles A, Lim J, Grey AC, Robertson K, Danesh-Meyer H, Palacios AG et al (2014) Alzheimer’s disease in the human eye. Clinical tests that identify ocular and visual information processing deficit as biomarkers. Alzheimers Dement 10(2):251–261. https://doi.org/10.1016/j.jalz.2013.06.004
Chiquita S, Rodrigues-Neves AC, Baptista FI, Carecho R, Moreira PI, Castelo-Branco M, Ambrosio AF (2019) The retina as a window or mirror of the brain changes detected in Alzheimer’s disease: critical aspects to unravel. Mol Neurobiol. 56:5416–5435. https://doi.org/10.1007/s12035-018-1461-6
Liew SC, Penfold PL, Provis JM, Madigan MC, Billson FA (1994) Modulation of MHC class II expression in the absence of lymphocytic infiltrates in Alzheimer’s retinae. J Neuropathol Exp Neurol 53(2):150–157. https://doi.org/10.1097/00005072-199403000-00006
Blanks JC, Schmidt SY, Torigoe Y, Porrello KV, Hinton DR, Blanks RH (1996) Retinal pathology in Alzheimer’s disease. II. Regional neuron loss and glial changes in GCL. Neurobiol Aging 17(3):385–395
Hopperton KE, Mohammad D, Trepanier MO, Giuliano V, Bazinet RP (2018) Markers of microglia in post-mortem brain samples from patients with Alzheimer’s disease: a systematic review. Mol Psychiatry 23(2):177–198. https://doi.org/10.1038/mp.2017.246
Hinton DR, Sadun AA, Blanks JC, Miller CA (1986) Optic-nerve degeneration in Alzheimer’s disease. N Engl J Med 315(8):485–487. https://doi.org/10.1056/NEJM198608213150804
Blanks JC, Torigoe Y, Hinton DR, Blanks RH (1991) Retinal degeneration in the macula of patients with Alzheimer’s disease. Ann N Y Acad Sci 640:44–46. https://doi.org/10.1111/j.1749-6632.1991.tb00188.x
Blanks JC, Torigoe Y, Hinton DR, Blanks RH (1996) Retinal pathology in Alzheimer’s disease. I. Ganglion cell loss in foveal/parafoveal retina. Neurobiol Aging 17(3):377–384
La Morgia C, Ross-Cisneros FN, Koronyo Y, Hannibal J, Gallassi R, Cantalupo G, Sambati L, Pan BX et al (2016) Melanopsin retinal ganglion cell loss in Alzheimer disease. Ann Neurol 79(1):90–109. https://doi.org/10.1002/ana.24548
Blanks JC, Hinton DR, Sadun AA, Miller CA (1989) Retinal ganglion cell degeneration in Alzheimer’s disease. Brain Res 501(2):364–372. https://doi.org/10.1016/0006-8993(89)90653-7
Koronyo-Hamaoui M, Koronyo Y, Ljubimov AV, Miller CA, Ko MK, Black KL, Schwartz M, Farkas DL (2011) Identification of amyloid plaques in retinas from Alzheimer’s patients and noninvasive in vivo optical imaging of retinal plaques in a mouse model. Neuroimage 54(Suppl 1):S204–S217. https://doi.org/10.1016/j.neuroimage.2010.06.020
Ho CY, Troncoso JC, Knox D, Stark W, Eberhart CG (2014) Beta-amyloid, phospho-tau and alpha-synuclein deposits similar to those in the brain are not identified in the eyes of Alzheimer’s and Parkinson’s disease patients. Brain Pathol 24(1):25–32. https://doi.org/10.1111/bpa.12070
Koronyo Y, Biggs D, Barron E, Boyer DS, Pearlman JA, Au WJ, Kile SJ, Blanco A et al (2017) Retinal amyloid pathology and proof-of-concept imaging trial in Alzheimer’s disease. JCI Insight 2(16). https://doi.org/10.1172/jci.insight.93621
Chiquita S, Ribeiro M, Castelhano J, Oliveira F, Sereno J, Batista M, Abrunhosa A, Rodrigues-Neves AC et al (2019) A longitudinal multimodal in vivo molecular imaging study of the 3xTg-AD mouse model shows progressive early hippocampal and taurine loss. Hum Mol Genet 28(13):2174–2188. https://doi.org/10.1093/hmg/ddz045
Chiquita S, Campos EJ, Castelhano J, Ribeiro M, Sereno J, Moreira PI, Castelo-Branco M, Ambrosio AF (2019) Retinal thinning of inner sub-layers is associated with cortical atrophy in a mouse model of Alzheimer’s disease: a longitudinal multimodal in vivo study. Alzheimers Res Ther 11(1):90. https://doi.org/10.1186/s13195-019-0542-8
Oddo S, Caccamo A, Shepherd JD, Murphy MP, Golde TE, Kayed R, Metherate R, Mattson MP et al (2003) Triple-transgenic model of Alzheimer’s disease with plaques and tangles: intracellular Abeta and synaptic dysfunction. Neuron 39(3):409–421
Casali BT, Landreth GE (2016) Abeta extraction from murine brain homogenates. Bio Protoc 6 (8). doi:10.21769/BioProtoc.1787
Baptista FI, Pinto MJ, Elvas F, Almeida RD, Ambrosio AF (2013) Diabetes alters KIF1A and KIF5B motor proteins in the hippocampus. PLoS One 8(6):e65515. https://doi.org/10.1371/journal.pone.0065515
George Paxinos KF (2008). The mouse brain in stereotaxic coordinates, compact. 3rd Edition. Elsevier Academic Press:256
Caetano L, Pinheiro H, Patricio P, Mateus-Pinheiro A, Alves ND, Coimbra B, Baptista FI, Henriques SN et al (2017) Adenosine A2A receptor regulation of microglia morphological remodeling-gender bias in physiology and in a model of chronic anxiety. Mol Psychiatry 22(7):1035–1043. https://doi.org/10.1038/mp.2016.173
Simoes-Henriques C, Mateus-Pinheiro M, Gaspar R, Pinheiro H, Mendes Duarte J, Baptista FI, Canas PM, Fontes-Ribeiro CA et al (2020) Microglia cytoarchitecture in the brain of adenosine A2A receptor knockout mice: brain region and sex specificities. Eur J Neurosci 51(6):1377–1387. https://doi.org/10.1111/ejn.14561
Duarte JM, Gaspar R, Caetano L, Patricio P, Soares-Cunha C, Mateus-Pinheiro A, Alves ND, Santos AR et al (2019) Region-specific control of microglia by adenosine A2A receptors: uncoupling anxiety and associated cognitive deficits in female rats. Glia 67(1):182–192. https://doi.org/10.1002/glia.23476
Mastrangelo MA, Bowers WJ (2008) Detailed immunohistochemical characterization of temporal and spatial progression of Alzheimer’s disease-related pathologies in male triple-transgenic mice. BMC Neurosci 9:81. https://doi.org/10.1186/1471-2202-9-81
Carvalho C, Cardoso S, Correia SC, Santos RX, Santos MS, Baldeiras I, Oliveira CR, Moreira PI (2012) Metabolic alterations induced by sucrose intake and Alzheimer’s disease promote similar brain mitochondrial abnormalities. Diabetes 61(5):1234–1242. https://doi.org/10.2337/db11-1186
Carvalho C, Machado N, Mota PC, Correia SC, Cardoso S, Santos RX, Santos MS, Oliveira CR et al (2013) Type 2 diabetic and Alzheimer’s disease mice present similar behavioral, cognitive, and vascular anomalies. J Alzheimers Dis 35(3):623–635. https://doi.org/10.3233/JAD-130005
Carvalho C, Santos MS, Oliveira CR, Moreira PI (2015) Alzheimer’s disease and type 2 diabetes-related alterations in brain mitochondria, autophagy and synaptic markers. Biochim Biophys Acta 1852(8):1665–1675. https://doi.org/10.1016/j.bbadis.2015.05.001
Yoon SS, Jo SA (2012) Mechanisms of amyloid-beta peptide clearance: potential therapeutic targets for Alzheimer’s disease. Biomol Ther (Seoul) 20(3):245–255. https://doi.org/10.4062/biomolther.2012.20.3.245
Ramanathan A, Nelson AR, Sagare AP, Zlokovic BV (2015) Impaired vascular-mediated clearance of brain amyloid beta in Alzheimer’s disease: the role, regulation and restoration of LRP1. Front Aging Neurosci 7:136. https://doi.org/10.3389/fnagi.2015.00136
Wildsmith KR, Holley M, Savage JC, Skerrett R, Landreth GE (2013) Evidence for impaired amyloid beta clearance in Alzheimer’s disease. Alzheimers Res Ther 5(4):33. https://doi.org/10.1186/alzrt187
Grimaldi A, Brighi C, Peruzzi G, Ragozzino D, Bonanni V, Limatola C, Ruocco G, Di Angelantonio S (2018) Inflammation, neurodegeneration and protein aggregation in the retina as ocular biomarkers for Alzheimer’s disease in the 3xTg-AD mouse model. Cell Death Dis 9(6):685. https://doi.org/10.1038/s41419-018-0740-5
Alexandrov PN, Pogue A, Bhattacharjee S, Lukiw WJ (2011) Retinal amyloid peptides and complement factor H in transgenic models of Alzheimer’s disease. Neuroreport 22(12):623–627. https://doi.org/10.1097/WNR.0b013e3283497334
Sprinkle TJ, McMorris FA, Yoshino J, DeVries GH (1985) Differential expression of 2':3'-cyclic nucleotide 3'-phosphodiesterase in cultured central, peripheral, and extraneural cells. Neurochem Res 10(7):919–931. https://doi.org/10.1007/BF00964629
Oddo S, Caccamo A, Cheng D, Jouleh B, Torp R, LaFerla FM (2007) Genetically augmenting tau levels does not modulate the onset or progression of Abeta pathology in transgenic mice. J Neurochem 102(4):1053–1063. https://doi.org/10.1111/j.1471-4159.2007.04607.x
Mondragon-Rodriguez S, Perry G, Luna-Munoz J, Acevedo-Aquino MC, Williams S (2014) Phosphorylation of tau protein at sites Ser(396-404) is one of the earliest events in Alzheimer’s disease and Down syndrome. Neuropathol Appl Neurobiol 40(2):121–135. https://doi.org/10.1111/nan.12084
Ujiie M, Dickstein DL, Carlow DA, Jefferies WA (2003) Blood–brain barrier permeability precedes senile plaque formation in an Alzheimer disease model. Microcirculation 10(6):463–470. https://doi.org/10.1038/sj.mn.7800212
Kumar-Singh S, Pirici D, McGowan E, Serneels S, Ceuterick C, Hardy J, Duff K, Dickson D et al (2005) Dense-core plaques in Tg2576 and PSAPP mouse models of Alzheimer’s disease are centered on vessel walls. Am J Pathol 167(2):527–543. https://doi.org/10.1016/S0002-9440(10)62995-1
Bien-Ly N, Boswell CA, Jeet S, Beach TG, Hoyte K, Luk W, Shihadeh V, Ulufatu S et al (2015) Lack of widespread BBB disruption in Alzheimer’s disease models: focus on therapeutic antibodies. Neuron 88(2):289–297. https://doi.org/10.1016/j.neuron.2015.09.036
Biron KE, Dickstein DL, Gopaul R, Jefferies WA (2011) Amyloid triggers extensive cerebral angiogenesis causing blood brain barrier permeability and hypervascularity in Alzheimer’s disease. PLoS One 6(8):e23789. https://doi.org/10.1371/journal.pone.0023789
Park SW, Kim JH, Mook-Jung I, Kim KW, Park WJ, Park KH, Kim JH (2014) Intracellular amyloid beta alters the tight junction of retinal pigment epithelium in 5XFAD mice. Neurobiol Aging 35(9):2013–2020. https://doi.org/10.1016/j.neurobiolaging.2014.03.008
Auld DS, Kornecook TJ, Bastianetto S, Quirion R (2002) Alzheimer’s disease and the basal forebrain cholinergic system: relations to beta-amyloid peptides, cognition, and treatment strategies. Prog Neurobiol 68(3):209–245
Girao da Cruz MT, Jordao J, Dasilva KA, Ayala-Grosso CA, Ypsilanti A, Weng YQ, Laferla FM, McLaurin J et al (2012) Early increases in soluble amyloid-beta levels coincide with cholinergic degeneration in 3xTg-AD mice. J Alzheimers Dis 32(2):267–272. https://doi.org/10.3233/JAD-2012-100732
Gao L, Chen X, Tang Y, Zhao J, Li Q, Fan X, Xu H, Yin ZQ (2015) Neuroprotective effect of memantine on the retinal ganglion cells of APPswe/PS1DeltaE9 mice and its immunomodulatory mechanisms. Exp Eye Res 135:47–58. https://doi.org/10.1016/j.exer.2015.04.013
Bravarenko NI, Onufriev MV, Stepanichev MY, Ierusalimsky VN, Balaban PM, Gulyaeva NV (2006) Caspase-like activity is essential for long-term synaptic plasticity in the terrestrial snail Helix. Eur J Neurosci 23(1):129–140. https://doi.org/10.1111/j.1460-9568.2005.04549.x
Li Z, Jo J, Jia JM, Lo SC, Whitcomb DJ, Jiao S, Cho K, Sheng M (2010) Caspase-3 activation via mitochondria is required for long-term depression and AMPA receptor internalization. Cell 141(5):859–871. https://doi.org/10.1016/j.cell.2010.03.053
D'Amelio M, Cavallucci V, Middei S, Marchetti C, Pacioni S, Ferri A, Diamantini A, De Zio D et al (2011) Caspase-3 triggers early synaptic dysfunction in a mouse model of Alzheimer’s disease. Nat Neurosci 14(1):69–76. https://doi.org/10.1038/nn.2709
Revilla S, Sunol C, Garcia-Mesa Y, Gimenez-Llort L, Sanfeliu C, Cristofol R (2014) Physical exercise improves synaptic dysfunction and recovers the loss of survival factors in 3xTg-AD mouse brain. Neuropharmacology 81:55–63. https://doi.org/10.1016/j.neuropharm.2014.01.037
Sun DS, Gao LF, Jin L, Wu H, Wang Q, Zhou Y, Fan S, Jiang X et al (2017) Fluoxetine administration during adolescence attenuates cognitive and synaptic deficits in adult 3xTgAD mice. Neuropharmacology 126:200–212. https://doi.org/10.1016/j.neuropharm.2017.08.037
Chen Y, Zhao Y, Dai CL, Liang Z, Run X, Iqbal K, Liu F, Gong CX (2014) Intranasal insulin restores insulin signaling, increases synaptic proteins, and reduces Abeta level and microglia activation in the brains of 3xTg-AD mice. Exp Neurol 261:610–619. https://doi.org/10.1016/j.expneurol.2014.06.004
Yao PJ, Bushlin I, Furukawa K (2005) Preserved synaptic vesicle recycling in hippocampal neurons in a mouse Alzheimer’s disease model. Biochem Biophys Res Commun 330(1):34–38. https://doi.org/10.1016/j.bbrc.2005.02.121
Baazaoui N, Flory M, Iqbal K (2017) Synaptic compensation as a probable cause of prolonged mild cognitive impairment in Alzheimer’s disease: implications from a transgenic mouse model of the disease. J Alzheimers Dis 56(4):1385–1401
Mukaetova-Ladinska EB, Garcia-Siera F, Hurt J, Gertz HJ, Xuereb JH, Hills R, Brayne C, Huppert FA et al (2000) Staging of cytoskeletal and beta-amyloid changes in human isocortex reveals biphasic synaptic protein response during progression of Alzheimer’s disease. Am J Pathol 157(2):623–636. https://doi.org/10.1016/s0002-9440(10)64573-7
Limon A, Reyes-Ruiz JM, Miledi R (2012) Loss of functional GABA(A) receptors in the Alzheimer diseased brain. Proc Natl Acad Sci U S A 109(25):10071–10076. https://doi.org/10.1073/pnas.1204606109
Bernareggi A, Duenas Z, Reyes-Ruiz JM, Ruzzier F, Miledi R (2007) Properties of glutamate receptors of Alzheimer’s disease brain transplanted to frog oocytes. Proc Natl Acad Sci U S A 104(8):2956–2960. https://doi.org/10.1073/pnas.0611513104
Gueli MC, Taibi G (2013) Alzheimer’s disease: amino acid levels and brain metabolic status. Neurol Sci 34(9):1575–1579. https://doi.org/10.1007/s10072-013-1289-9
Villette V, Dutar P (2017) GABAergic microcircuits in Alzheimer’s disease models. Curr Alzheimer Res 14(1):30–39
Huang D, Liu D, Yin J, Qian T, Shrestha S, Ni H (2017) Glutamate–glutamine and GABA in brain of normal aged and patients with cognitive impairment. Eur Radiol 27(7):2698–2705. https://doi.org/10.1007/s00330-016-4669-8
Calvo-Flores Guzman B, Vinnakota C, Govindpani K, Waldvogel HJ, Faull RLM, Kwakowsky A (2018) The GABAergic system as a therapeutic target for Alzheimer’s disease. J Neurochem 146(6):649–669. https://doi.org/10.1111/jnc.14345
Hascup KN, Hascup ER (2015) Altered neurotransmission prior to cognitive decline in AbetaPP/PS1 mice, a model of Alzheimer’s disease. J Alzheimers Dis 44(3):771–776. https://doi.org/10.3233/JAD-142160
Silva AC, Lemos C, Goncalves FQ, Pliassova AV, Machado NJ, Silva HB, Canas PM, Cunha RA et al (2018) Blockade of adenosine A2A receptors recovers early deficits of memory and plasticity in the triple transgenic mouse model of Alzheimer’s disease. Neurobiol Dis 117:72–81. https://doi.org/10.1016/j.nbd.2018.05.024
Dzamba D, Harantova L, Butenko O, Anderova M (2016) Glial cells—the key elements of Alzheimer’s disease. Curr Alzheimer Res 13(8):894–911
Chidlow G, Wood JP, Manavis J, Finnie J, Casson RJ (2017) Investigations into retinal pathology in the early stages of a mouse model of Alzheimer’s disease. J Alzheimers Dis 56(2):655–675. https://doi.org/10.3233/JAD-160823
Edwards MM, Rodriguez JJ, Gutierrez-Lanza R, Yates J, Verkhratsky A, Lutty GA (2014) Retinal macroglia changes in a triple transgenic mouse model of Alzheimer’s disease. Exp Eye Res 127:252–260. https://doi.org/10.1016/j.exer.2014.08.006
Joly S, Lamoureux S, Pernet V (2017) Nonamyloidogenic processing of amyloid beta precursor protein is associated with retinal function improvement in aging male APPswe/PS1DeltaE9 mice. Neurobiol Aging 53:181–191. https://doi.org/10.1016/j.neurobiolaging.2017.02.004
Tay TL, Savage JC, Hui CW, Bisht K, Tremblay ME (2017) Microglia across the lifespan: from origin to function in brain development, plasticity and cognition. J Physiol 595(6):1929–1945. https://doi.org/10.1113/JP272134
Rodriguez JJ, Noristani HN, Hilditch T, Olabarria M, Yeh CY, Witton J, Verkhratsky A (2013) Increased densities of resting and activated microglia in the dentate gyrus follow senile plaque formation in the CA1 subfield of the hippocampus in the triple transgenic model of Alzheimer’s disease. Neurosci Lett 552:129–134. https://doi.org/10.1016/j.neulet.2013.06.036
Salobrar-Garcia E, Rodrigues-Neves AC, Ramirez AI, de Hoz R, Fernandez-Albarral JA, Lopez-Cuenca I, Ramirez JM, Ambrosio AF et al (2020) Microglial activation in the retina of a triple-transgenic Alzheimer’s disease mouse model (3xTg-AD). Int J Mol Sci 21(3). https://doi.org/10.3390/ijms21030816
Davies DS, Ma J, Jegathees T, Goldsbury C (2017) Microglia show altered morphology and reduced arborization in human brain during aging and Alzheimer’s disease. Brain Pathol 27(6):795–808. https://doi.org/10.1111/bpa.12456
Funding
Santa Casa Mantero Belard Award 2015 (MB-1049-2015), Foundation for Science and Technology (PEst UID/NEU/04539/2013 and UID/NEU/04539/2019: CNC.IBILI; PEst UIDB/04539/2020 and UIDP/04539/2020: CIBB), COMPETE-FEDER (POCI-01-0145-FEDER-007440), and Centro 2020 Regional Operational Programme (CENTRO-01-0145-FEDER-000008: BrainHealth 2020).
Author information
Authors and Affiliations
Contributions
ACRN, RC, and AFA conceived and designed the experiments. ACRN, RC, SCC, CC, EJC, and FIB performed the experiments. ACRN, RC, SCC, CC, EJC, FIB, PIM, and AFA analyzed the results. PIM and AFA contributed with reagents/materials/analysis tools. ACRN wrote the first draft of the manuscript, and all authors have read and approved the final version.
Corresponding author
Ethics declarations
Ethics Approval
All experiments using animals were approved by the Animal Welfare (Órgão Responsável pelo Bem-Estar Animal - ORBEA 16/2015) of the Coimbra Institute for Clinical and Biomedical research (iCBR), Faculty of Medicine, University of Coimbra, and by Direção Geral de Alimentação e Veterinária (DGAV 0421/000/000/2015) and conducted in accordance with the European Community directive guidelines for the use of animals in laboratory (2010/63/EU) transposed to the Portuguese law in 2013 (Decreto-Lei 113/2013), and in agreement with the Association for Research in Vision and Ophthalmology statement for animal use.
Consent to Participate
Not applicable.
Consent for Publication
All authors have reviewed and approved the manuscript.
Conflict of Interest
The authors declare no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supporting Information
Online Resource 1
includes table 1 and table 2. (DOCX 16 kb)
Online Resource 2
can be found on online version. (AVI 49152 kb)
Rights and permissions
About this article
Cite this article
Rodrigues-Neves, A.C., Carecho, R., Correia, S.C. et al. Retina and Brain Display Early and Differential Molecular and Cellular Changes in the 3xTg-AD Mouse Model of Alzheimer’s Disease. Mol Neurobiol 58, 3043–3060 (2021). https://doi.org/10.1007/s12035-021-02316-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-021-02316-x