Abstract
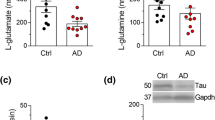
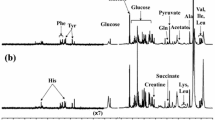
To study brain free amino acids and their relation with dementia we measured, by high-performance liquid chromatography (HPLC), the concentration of eight free amino acids, amines and related compounds. We used temporal cortex (TC) samples obtained from 13 Alzheimer’s disease (AD) patients and an equal number of age-matched controls (AC). The patterns of free amino acids, amines and related compounds showed significant quantitative changes in AD conditions with respect to healthy ones. In Alzheimer patients, lower levels of GABA were found in the TC (−57 %). Amino acids glutamate (Glu), and aspartate (Asp) concentrations, also appeared significantly reduced in the TC of AD patients (Glu: −30 %; Asp: −40 %) when compared with controls. The significant gap between methionine (Met: −30 %) and cystathionine (Cysta: +60 %) levels in TC of AD people to controls, might suggest an under/over activity of the transmethylation and transsulphuration pathways, respectively. Glutamine (Gln) and Urea were an exception to this trend because their content was higher in AD patients than in controls. Albeit these compounds may have particular physiological roles, including the possible mediation of synaptic transmission, changes in amino acid levels and related compounds (detected in steady state) suggest a modified metabolic status in brains of AD patients that reveals a reduced function of synaptic transmission. Because several evidences show that patients might display quite different concentrations of neurotransmitters in brain areas, assessing metabolites in different and well-characterized AD stages should be investigated further.
Similar content being viewed by others
References
Hyman BT (1996) Anatomy of pathological alterations in Alzheimer’s disease. In: Wasco W, Tanzi RE (eds) Molecular mechanisms of dementia. Humana Press Inc., Totowa, pp 219–223
Nitsch RM (1996) From acetylcholine to amyloid: neurotransmitters and the pathology of Alzheimer’s disease. Neurodegeneration 5:477–482
Bowen DM, Allen SJ, Benton JS, Goodhardt MJ, Haan EA, Palmer AM, Sims NR, Smith CCT, Spillane JA, Esiri MM, Neary D, Snowdon JS, Wilcok GK, Davison AN (1983) Biochemical assessment of serotonergic and cholinergic dysfunction and cerebral atrophy in Alzheimer’s disease. J Neurochem 41:266–272
Yuan J, Yankner BA (2000) Apoptosis in the nervous system. Nature 407:802–809
Hardy JA, Higgins GA (1992) Alzheimer’s disease: the amyloid cascade hypothesis. Science 25:184–185
Tarbit I, Perry EK, Perry RH, Blessed G, Tomlinson BE (1980) Hippocampal free amino acids in Alzheimer’s disease. J Neurochem 35:1246–1249
Tierney MC, Fisher RH, Lewis AJ, Zorzitto ML, Snow WG, Reid DW, Nieuwstraten P (1988) The NINCDS-ADRDA work group criteria for the clinical diagnosis of probable Alzheimer’s disease. Neurology 38:359–364
Mirra SS, Heyman A, McKill D, Sumi SM, Crain BS, Brownlee LM, Vogel SS, Hughes JP, Van Bele G, Berg L (1991) The consortium to establish a registry for Alzheimer disease (CERAD II). Standardisation of the neuropathological assessment of Alzheimer’s disease. Neurology 41:479–486
Borum PR (1985) Manual for amino acid analysis of physiological samples. Atlanta GA American Association for Clinical Chemistry 37th National Meeting
Smith CD, Carney JM, Starke-Reed PE, Oliver CN, Stadtman ER, Floyd RA, Markesbery WR (1991) Excess brain protein oxidation and enzyme disfunction in normal aging and in Alzheimer disease. Proc Natl Acad Sci USA 88:10540–10543
Cantoni L (1975) Biological methylation: selected aspects. Annu Rev Biochem 44:435–451
Morrison LD, Bergeron C, Kish SJ (1993) Brain S-Adenosylmethionine decarboxylase activity is increased in Alzheimer’s disease. Neurosci Lett 154:141–144
Bottiglieri T, Godfrey P, Flynn T, Carney MWP, Toone BK, Reynolds EH (1990) Cerebrospinal fluid S-adenosylmethionine in depression and dementia: effects of treatment with parenteral and oral S-adenosylmethionine. J Neurol Neurosurg Pschiatry 53:1096–1098
Martínez-Chantar ML, Latasa MU, Varela-Rey MLuC, García-Trevijano ER, Mato JM, Avila MA (2003) l-Methionine availability regulates expression of the methionine adenosyltransferase 2A gene in human hepatocarcinoma cells. J Biol Chem 278:19885–19890
Tallan HH, Moore S, Stein WH (1958) l-Cystathionine in human brain. J Biol Chem 230:707–716
Okumura N, Kawai K (1961) Nitrogen metabolism of the brain. Folia Psychiatr Neurol Jpn 15:133–146
Dringer R (2000) Metabolism and function of glutathione in brain. Prog Neurol 62:649–671
Vitvitsky V, Thomas M, Ghorpade A, Gendelman HE, Banerjee R (2006) A functional trassulfuration pathaway in the brain links to glutathione homeostasis. J Biol Chem 281:35785–35793
Nussler AK, Billiar TR, Liu Z–Z, Morris MJ (1994) Coinduction of nitric oxide synthase and argininosuccinate synthetase in a murine macrophage cell line. J Biol Chem 269:1257–1261
Aksenov M, Aksenova M, Butterfield DA, Markesbery WR (2000) Oxidative modification of creatine kinase bb in Alzheimer’s disease brain. J Neurochem 74:2520–2527
Bowen DM, Smith CD, White P, Dawson AN (1976) Neurotransmitter related enzymes and indices of hypoxia in senile dementia and other abiotrophies. Brain 99:459–496
Ramonet D, Rodriguez MJ, Fredriksson K, Bernal F, Mahy N (2004) In vivo neuroprotective adaptation of the Glutamate/Glutamine cycle to neuronal death. Hippocampus 14:586–594
Chen J, Herrup K (2012) Glutamine act as a neuroprotectant against DNA damage, be-amyloid and H2O2-induced stress. PLoS One 7(3):e33177
Hamberger A, Jacobson I, Lindroth P, Mopper K, Nystrom B, Sandberg M, Molin S-O, Svanberg U (1981) Neuron-glia interactions in the biosynthesis and release of transmitter amino acids: in amino acid neurotransmitters. In: De Feudis FV, Mandel (eds) Raven Press, New York, pp 509–518
Procter AW, Palmer AM, Francis PT, Lowe SL, Neary D, Murphy E, Doshi R, Bowen DM (1988) Evidence of glutamatergic denervation and possible abnormal metabolism in Alzheimer’s disease. J Neurochem 50:790–802
Fayed N, Modrego PJ, Rojas-Salinas G, Aguilar K (2011) Brain glutamate levels are decreased in Alzheimer’s disease: a magnetic resonance spectroscopy study. Am J Alzheimers Dis Other Dem 26(6):450–456
Lee J, Ryu H, Ferrante RJ, Morris SM, Ratan RR (2002) Translational control of inducible nitric oxide synthase expression by arginine can explain the arginine paradox. PNAS 100(8):4843–4848
Auvinem M, Paasinem A, Andersson LC, Holta E (1992) Ornithine decarboxylase activity is critical for cell transformation. Nature 360:355–358
Raina AK, Monteiro MJ, Mc Shea A, Smith MA (1999) The role of cell cycle mediated events in Alzheimer’s disease. Int Exp Path 80:71–76
LeBlanc A, Li H, Goodyer C, Bergeron C, Hammond J (1999) Caspase-6 role in apoptosis of human neurons, amyloidogenesis, and Alzheimer’s disease. J Biol Chem 274:23426–23436
Gervais FG, Xu D, Robertson GS, Vaillancourt JP, Zhu Y, Huang J, LeBlanc A, Smith D, Rigby M, Shearman MS, Clarke EE, Zheng H, Van Der Ploeg LHT, Ruffolo SC, Thornberry NA, Xanthoudakis S, Zamboni RJ, Roy S, Nicholson DW (1999) Involvement of caspases in proteolytic cleavage of Alzheimer’s amyloid-β precursor protein and amyloidogenic Aβ peptide formation. Cell 97(3):395–406
Fafournoux P, Bruhat A, Jousse C (2000) Amino acid regulation of gene expression. Biochem J 351:1–12
Jousse C, Averous J, Bruhat A, Carraro V, Mordier S, Fafournoux P (2004) Amino acids as regulators of gene expression: molecular mechanisms. BBRC 313:447–452
Bruhat A, Jousse C, Carraro V, Reimold AM, Ferrara M, Fafournoux P (2000) Amino acids control mammalian gene trascription: activating transcription factor 2 is essential for the amino acid responsiveness of the CHOP promoter. Mol Cell Biol 20:7192–7204
Chen H, Pan Y-X, Dudenhausen EE, Kilberg MS (2004) Amino acid deprivation induces the trascription rate of the human asparagine synthetase gene through a timed program of expression and promoter binding of nutrient-responsive basic region/leucine zipper transcription factors as well as localised histone acetylation. J Biol Chem 279:50829–50839
Shaheen HH, Horetsky RL, Kimball SR, Murthi A, Jefferson LS, Hopper AK (2007) Retrograde nuclear accumulation of cytoplasmic tRNA in rat hepatoma cells in response to amino acid deprivation. PNAS 104:8845–8850
Van Eldik LJ (2001) Glia and Alzheimer’s disease. Neurochem Int 39:329–331
Acknowledgments
We thank Prof Gert Lubec for critical reading of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gueli, M.C., Taibi, G. Alzheimer’s disease: amino acid levels and brain metabolic status. Neurol Sci 34, 1575–1579 (2013). https://doi.org/10.1007/s10072-013-1289-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10072-013-1289-9