Abstract
Recent evidence indicates that disruption of epidermal growth factor (EGF) signaling by mutant huntingtin (polyQ-htt) may contribute to the onset of behavioral deficits observed in Huntington’s disease (HD) through a variety of mechanisms, including cerebrovascular dysfunction. Yet, whether EGF signaling modulates the development of HD pathology and the associated behavioral impairments remain unclear. To gain insight on this issue, we used the R6/2 mouse model of HD to assess the impact of chronic EGF treatment on behavior, and cerebrovascular and cortical neuronal functions. We found that bi-weekly treatment with a low dose of EGF (300 µg/kg, i.p.) for 6 weeks was sufficient to effectively improve motor behavior in R6/2 mice and diminish mortality, compared to vehicle-treated littermates. These beneficial effects of EGF treatment were dissociated from changes in cerebrovascular leakiness, a result that was surprising given that EGF ameliorates this deficit in other neurodegenerative diseases. Rather, the beneficial effect of EGF on R6/2 mice behavior was concomitant with a marked amelioration of cortical GABAergic function. As GABAergic transmission in cortical circuits is disrupted in HD, these novel data suggest a potential mechanistic link between deficits in EGF signaling and GABAergic dysfunction in the progression of HD.




Similar content being viewed by others
Availability of Data and Materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- aCSF:
-
Artificial cerebrospinal fluid
- EGF:
-
Epidermal growth factor
- GAD:
-
Glutamate decarboxylase
- HD:
-
Huntington’s disease
- htt:
-
Huntingtin protein
- polyQ-htt:
-
Mutant htt
- IHC:
-
Immunohistochemical
- IPSC:
-
Inhibitory postsynaptic currents
- KHC:
-
Kinesin heavy chain
- PFC:
-
Prefrontal cortex
- R6/2 mice:
-
Mice that express the 5′ end of the human HD gene containing 160 ± 5 poly Q
- TBSX:
-
TBS containing 0.25% triton X-100
References
Gusella JF, Wexler NS, Conneally PM, Naylor SL, Anderson MA, Tanzi RE, Watkins PC, Ottina K et al (1983) A polymorphic DNA marker genetically linked to Huntington’s disease. Nature 306(5940):234–238
Han I, You Y, Kordower JH, Brady ST, Morfini GA (2010) Differential vulnerability of neurons in Huntington’s disease: the role of cell type-specific features. J Neurochem 113(5):1073–1091. https://doi.org/10.1111/j.1471-4159.2010.06672.x
Roze E, Bonnet C, Betuing S, Caboche J (2010) Huntington’s disease. Adv Exp Med Biol 685:45–63
Bodai L, Marsh JL (2012) A novel target for Huntington’s disease: ERK at the crossroads of signaling. The ERK signaling pathway is implicated in Huntington's disease and its upregulation ameliorates pathology. Bioessays 34(2):142–148
Song C, Perides G, Liu YF (2002) Expression of full-length polyglutamine-expanded huntingtin disrupts growth factor receptor signaling in rat pheochromocytoma (PC12) cells. J Biol Chem 277(8):6703–6707. https://doi.org/10.1074/jbc.M110338200
Melone MA, Calarco A, Petillo O, Margarucci S, Colucci-D'Amato L, Galderisi U, Koverech G, Peluso G (2013) Mutant huntingtin regulates EGF receptor fate in non-neuronal cells lacking wild-type protein. Biochim Biophys Acta 1832(1):105–113. https://doi.org/10.1016/j.bbadis.2012.09.001
Lievens JC, Rival T, Iche M, Chneiweiss H, Birman S (2005) Expanded polyglutamine peptides disrupt EGF receptor signaling and glutamate transporter expression in Drosophila. Hum Mol Genet 14(5):713–724. https://doi.org/10.1093/hmg/ddi067
Lin CY, Hsu YH, Lin MH, Yang TH, Chen HM, Chen YC, Hsiao HY, Chen CC et al (2013) Neurovascular abnormalities in humans and mice with Huntington’s disease. Exp Neurol 250:20–30. https://doi.org/10.1016/j.expneurol.2013.08.019
Di Pardo A, Amico E, Scalabri F, Pepe G, Castaldo S, Elifani F, Capocci L, De Sanctis C et al (2017) Impairment of blood-brain barrier is an early event in R6/2 mouse model of Huntington disease. Sci Rep 7:41316. https://doi.org/10.1038/srep41316
Hsiao HY, Chen YC, Huang CH, Chen CC, Hsu YH, Chen HM, Chiu FL, Kuo HC et al (2015) Aberrant astrocytes impair vascular reactivity in Huntington disease. Ann Neurol 78(2):178–192. https://doi.org/10.1002/ana.24428
Estrada-Sanchez AM, Rebec GV (2013) Role of cerebral cortex in the neuropathology of Huntington’s disease. Front Neural Circuits 7:19. https://doi.org/10.3389/fncir.2013.00019
Tang Y, Ye M, Du Y, Qiu X, Lv X, Yang W, Luo J (2015) EGFR signaling upregulates surface expression of the GluN2B-containing NMDA receptor and contributes to long-term potentiation in the hippocampus. Neuroscience 304:109–121. https://doi.org/10.1016/j.neuroscience.2015.07.021
Terlau H, Seifert W (1989) Influence of epidermal growth factor on long-term potentiation in the hippocampal slice. Brain Res 484(1–2):352–356
Namba H, Zheng Y, Abe Y, Nawa H (2009) Epidermal growth factor administered in the periphery influences excitatory synaptic inputs onto midbrain dopaminergic neurons in postnatal mice. Neuroscience 158(4):1731–1741. https://doi.org/10.1016/j.neuroscience.2008.10.057
Abe K, Saito H (1992) Epidermal growth factor selectively enhances NMDA receptor-mediated increase of intracellular Ca2+ concentration in rat hippocampal neurons. Brain Res 587(1):102–108
Tohmi M, Tsuda N, Mizuno M, Takei N, Frankland PW, Nawa H (2005) Distinct influences of neonatal epidermal growth factor challenge on adult neurobehavioral traits in four mouse strains. Behav Genet 35(5):615–629. https://doi.org/10.1007/s10519-005-5357-7
Futamura T, Kakita A, Tohmi M, Sotoyama H, Takahashi H, Nawa H (2003) Neonatal perturbation of neurotrophic signaling results in abnormal sensorimotor gating and social interaction in adults: implication for epidermal growth factor in cognitive development. Mol Psychiatry 8(1):19–29. https://doi.org/10.1038/sj.mp.4001138
Nagano T, Namba H, Abe Y, Aoki H, Takei N, Nawa H (2007) In vivo administration of epidermal growth factor and its homologue attenuates developmental maturation of functional excitatory synapses in cortical GABAergic neurons. Eur J Neurosci 25(2):380–390. https://doi.org/10.1111/j.1460-9568.2007.05297.x
Namba H, Nagano T, Jodo E, Eifuku S, Horie M, Takebayashi H, Iwakura Y, Sotoyama H et al (2017) Epidermal growth factor signals attenuate phenotypic and functional development of neocortical GABA neurons. J Neurochem 142:886–900. https://doi.org/10.1111/jnc.14097
Sotoyama H, Namba H, Chiken S, Nambu A, Nawa H (2013) Exposure to the cytokine EGF leads to abnormal hyperactivity of pallidal GABA neurons: implications for schizophrenia and its modeling. J Neurochem 126(4):518–528. https://doi.org/10.1111/jnc.12223
Rattray I, Smith E, Gale R, Matsumoto K, Bates GP, Modo M (2013) Correlations of behavioral deficits with brain pathology assessed through longitudinal MRI and histopathology in the R6/2 mouse model of HD. PLoS One 8(4):e60012. https://doi.org/10.1371/journal.pone.0060012
Thomas R, Morris AWJ, Tai LM (2017) Epidermal growth factor prevents APOE4-induced cognitive and cerebrovascular deficits in female mice. Heliyon 3(6):e00319. https://doi.org/10.1016/j.heliyon.2017.e00319
Thomas R, Zuchowska P, Morris AW, Marottoli FM, Sunny S, Deaton R, Gann PH, Tai LM (2016) Epidermal growth factor prevents APOE4 and amyloid-beta-induced cognitive and cerebrovascular deficits in female mice. Acta Neuropathol Commun 4(1):111. https://doi.org/10.1186/s40478-016-0387-3
Gatto RG, Chu Y, Ye AQ, Price SD, Tavassoli E, Buenaventura A, Brady ST, Magin RL et al (2015) Analysis of YFP(J16)-R6/2 reporter mice and postmortem brains reveals early pathology and increased vulnerability of callosal axons in Huntington’s disease. Hum Mol Genet 24(18):5285–5298. https://doi.org/10.1093/hmg/ddv248
Marottoli FM, Katsumata Y, Koster KP, Thomas R, Fardo DW, Tai LM (2017) Peripheral inflammation, apolipoprotein E4, and amyloid-beta interact to induce cognitive and cerebrovascular dysfunction. ASN Neuro 9 (4):1759091417719201. doi:https://doi.org/10.1177/1759091417719201
Cass DK, Flores-Barrera E, Thomases DR, Vital WF, Caballero A, Tseng KY (2014) CB1 cannabinoid receptor stimulation during adolescence impairs the maturation of GABA function in the adult rat prefrontal cortex. Mol Psychiatry 19(5):536–543. https://doi.org/10.1038/mp.2014.14
Flores-Barrera E, Thomases DR, Cass DK, Bhandari A, Schwarcz R, Bruno JP, Tseng KY (2017) Preferential disruption of prefrontal GABAergic function by nanomolar concentrations of the alpha7nACh negative modulator kynurenic acid. J Neurosci 37(33):7921–7929. https://doi.org/10.1523/JNEUROSCI.0932-17.2017
Menalled L, El-Khodor BF, Patry M, Suarez-Farinas M, Orenstein SJ, Zahasky B, Leahy C, Wheeler V et al (2009) Systematic behavioral evaluation of Huntington’s disease transgenic and knock-in mouse models. Neurobiol Dis 35(3):319–336. https://doi.org/10.1016/j.nbd.2009.05.007
Berlanga-Acosta J, Gavilondo-Cowley J, Lopez-Saura P, Gonzalez-Lopez T, Castro-Santana MD, Lopez-Mola E, Guillen-Nieto G, Herrera-Martinez L (2009) Epidermal growth factor in clinical practice—a review of its biological actions, clinical indications and safety implications. Int Wound J 6(5):331–346. https://doi.org/10.1111/j.1742-481X.2009.00622.x
Gu X, Li C, Wei W, Lo V, Gong S, Li SH, Iwasato T, Itohara S et al (2005) Pathological cell-cell interactions elicited by a neuropathogenic form of mutant huntingtin contribute to cortical pathogenesis in HD mice. Neuron 46(3):433–444. https://doi.org/10.1016/j.neuron.2005.03.025
Dougherty SE, Hollimon JJ, McMeekin LJ, Bohannon AS, West AB, Lesort M, Hablitz JJ, Cowell RM (2014) Hyperactivity and cortical disinhibition in mice with restricted expression of mutant huntingtin to parvalbumin-positive cells. Neurobiol Dis 62:160–171. https://doi.org/10.1016/j.nbd.2013.10.002
Spampanato J, Gu X, Yang XW, Mody I (2008) Progressive synaptic pathology of motor cortical neurons in a BAC transgenic mouse model of Huntington’s disease. Neuroscience 157(3):606–620. https://doi.org/10.1016/j.neuroscience.2008.09.020
Skotte NH, Andersen JV, Santos A, Aldana BI, Willert CW, Norremolle A, Waagepetersen HS, Nielsen ML (2018) Integrative characterization of the R6/2 mouse model of Huntington’s disease reveals dysfunctional astrocyte metabolism. Cell Rep 23(7):2211–2224. https://doi.org/10.1016/j.celrep.2018.04.052
Qi J, Kim M, Sanchez R, Ziaee SM, Kohtz JD, Koh S (2018) Enhanced susceptibility to stress and seizures in GAD65 deficient mice. PLoS One 13(1):e0191794. https://doi.org/10.1371/journal.pone.0191794
Gourfinkel-An I, Parain K, Hartmann A, Mangiarini L, Brice A, Bates G, Hirsch EC (2003) Changes in GAD67 mRNA expression evidenced by in situ hybridization in the brain of R6/2 transgenic mice. J Neurochem 86(6):1369–1378
Walker AG, Miller BR, Fritsch JN, Barton SJ, Rebec GV (2008) Altered information processing in the prefrontal cortex of Huntington’s disease mouse models. J Neurosci 28(36):8973–8982. https://doi.org/10.1523/JNEUROSCI.2804-08.2008
Bristow LJ, Hutson PH, Thorn L, Tricklebank MD (1993) The glycine/NMDA receptor antagonist, R-(+)-HA-966, blocks activation of the mesolimbic dopaminergic system induced by phencyclidine and dizocilpine (MK-801) in rodents. Br J Pharmacol 108(4):1156–1163
Takahata R, Moghaddam B (2003) Activation of glutamate neurotransmission in the prefrontal cortex sustains the motoric and dopaminergic effects of phencyclidine. Neuropsychopharmacology 28(6):1117–1124. https://doi.org/10.1038/sj.npp.1300127
Sokolowski JD, Salamone JD (1994) Effects of dopamine depletions in the medial prefrontal cortex on DRL performance and motor activity in the rat. Brain Res 642(1–2):20–28
Jinks AL, McGregor IS (1997) Modulation of anxiety-related behaviours following lesions of the prelimbic or infralimbic cortex in the rat. Brain Res 772(1–2):181–190
Jentsch JD, Tran A, Taylor JR, Roth RH (1998) Prefrontal cortical involvement in phencyclidine-induced activation of the mesolimbic dopamine system: behavioral and neurochemical evidence. Psychopharmacology 138(1):89–95
Drouin-Ouellet J, Sawiak SJ, Cisbani G, Lagace M, Kuan WL, Saint-Pierre M, Dury RJ, Alata W et al (2015) Cerebrovascular and blood-brain barrier impairments in Huntington's disease: potential implications for its pathophysiology. Ann Neurol 78(2):160–177. https://doi.org/10.1002/ana.24406
Lim RG, Quan C, Reyes-Ortiz AM, Lutz SE, Kedaigle AJ, Gipson TA, Wu J, Vatine GD et al (2017) Huntington’s disease iPSC-derived brain microvascular endothelial cells reveal WNT-mediated angiogenic and blood-brain barrier deficits. Cell Rep 19(7):1365–1377. https://doi.org/10.1016/j.celrep.2017.04.021
Hsu YT, Chang YG, Chern Y (2018) Insights into GABAAergic system alteration in Huntington’s disease. Open Biol 8(12):180165. https://doi.org/10.1098/rsob.180165
Philpott AL, Cummins TDR, Bailey NW, Churchyard A, Fitzgerald PB, Georgiou-Karistianis N (2016) Cortical inhibitory deficits in premanifest and early Huntington’s disease. Behav Brain Res 296:311–317. https://doi.org/10.1016/j.bbr.2015.09.030
Cummings DM, Andre VM, Uzgil BO, Gee SM, Fisher YE, Cepeda C, Levine MS (2009) Alterations in cortical excitation and inhibition in genetic mouse models of Huntington’s disease. J Neurosci 29(33):10371–10386. https://doi.org/10.1523/JNEUROSCI.1592-09.2009
Spokes EG, Garrett NJ, Rossor MN, Iversen LL (1980) Distribution of GABA in post-mortem brain tissue from control, psychotic and Huntington’s chorea subjects. J Neurol Sci 48(3):303–313
Perry TL, Hansen S, Kloster M (1973) Huntington’s chorea. Deficiency of gamma-aminobutyric acid in brain. N Engl J Med 288(7):337–342. https://doi.org/10.1056/NEJM197302152880703
Reynolds GP, Pearson SJ (1990) Brain GABA levels in asymptomatic Huntington’s disease. N Engl J Med 323(10):682–683. https://doi.org/10.1056/NEJM199009063231014
Reynolds GP, Pearson SJ (1987) Decreased glutamic acid and increased 5-hydroxytryptamine in Huntington's disease brain. Neurosci Lett 78(2):233–238
Iwakura Y, Nawa H (2013) ErbB1-4-dependent EGF/neuregulin signals and their cross talk in the central nervous system: pathological implications in schizophrenia and Parkinson’s disease. Front Cell Neurosci 7:4. https://doi.org/10.3389/fncel.2013.00004
Sanchez-Huertas C, Rico B (2011) CREB-dependent regulation of GAD65 transcription by BDNF/TrkB in cortical interneurons. Cereb Cortex 21(4):777–788. https://doi.org/10.1093/cercor/bhq150
Chou CC, Modi JP, Wang CY, Hsu PC, Lee YH, Huang KF, Wang AH, Nan C et al (2017) Activation of brain L-glutamate decarboxylase 65 isoform (GAD65) by phosphorylation at threonine 95 (T95). Mol Neurobiol 54(2):866–873. https://doi.org/10.1007/s12035-015-9633-0
Rush DB, Leon RT, McCollum MH, Treu RW, Wei J (2012) Palmitoylation and trafficking of GAD65 are impaired in a cellular model of Huntington’s disease. Biochem J 442(1):39–48. https://doi.org/10.1042/BJ20110679
Morfini GA, You YM, Pollema SL, Kaminska A, Liu K, Yoshioka K, Bjorkblom B, Coffey ET et al (2009) Pathogenic huntingtin inhibits fast axonal transport by activating JNK3 and phosphorylating kinesin. Nat Neurosci 12(7):864–871. https://doi.org/10.1038/nn.2346
Song M, Messing RO (2005) Protein kinase C regulation of GABAA receptors. Cell Mol Life Sci 62(2):119–127. https://doi.org/10.1007/s00018-004-4339-x
Bell-Horner CL, Dohi A, Nguyen Q, Dillon GH, Singh M (2006) ERK/MAPK pathway regulates GABAA receptors. J Neurobiol 66(13):1467–1474. https://doi.org/10.1002/neu.20327
Lund IV, Hu Y, Raol YH, Benham RS, Faris R, Russek SJ, Brooks-Kayal AR (2008) BDNF selectively regulates GABAA receptor transcription by activation of the JAK/STAT pathway. Sci Signal 1(41):ra9. https://doi.org/10.1126/scisignal.1162396
Li SH, Yu ZX, Li CL, Nguyen HP, Zhou YX, Deng C, Li XJ (2003) Lack of huntingtin-associated protein-1 causes neuronal death resembling hypothalamic degeneration in Huntington's disease. J Neurosci 23(17):6956–6964
Giampa C, Montagna E, Dato C, Melone MA, Bernardi G, Fusco FR (2013) Systemic delivery of recombinant brain derived neurotrophic factor (BDNF) in the R6/2 mouse model of Huntington’s disease. PLoS One 8(5):e64037. https://doi.org/10.1371/journal.pone.0064037
Acknowledgments
We would like to thank Norma Hernandez for technical assistance with mouse breeding and UL1TR002003, which allowed the use of the Keyence microscope.
Funding
Supported by National Institutes of Health Grants (R01AG061114, R21AG053876, and R21AG061715 to L.M.T; R21NS096642 to G.A.M.), University of Illinois at Chicago institutional start-up funds (L.M.T), and CHDI research contract No. A-11014 (G.A.M and S.T.B).
Author information
Authors and Affiliations
Contributions
L.M.T. and K.Y.T. wrote the manuscript and prepared the figures. L.M.T, F.M.M., G.A.M., S.T.B., and K.Y.T. designed and supervised the study. F.M.M., L.M.T., P.M., R.P., S.Z., K.D.F., G.K.E., and N.H. conducted behavior testing, biochemical, and immunohistochemical analyses. K.Y.T. and E.F. designed and performed the electrophysiological experiments and data analyses. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare they have no conflict of interest.
Research Involving Human Participants and/or Animals
This article does not contain any studies with human participants performed by any of the authors.
Ethical Approval
All procedures follow the UIC Institutional Animal Care and Use Committee protocols.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
Supplementary Fig. 1
Sex does not modulate open-field or rotarod performance in R6/2 mice. Ten/eleven-week-old male and female, wild-type (WT), and R6/2 mice were tested for motor behavior. Compared to WT mice, R6/2 mice displayed a impaired performance in the rotarod test and b hypoactivity, lower average speed, and higher number of stops in the open-field behavioral test. Rotarod: F(2, 23) = 70.63. Open-field distance: F(2, 23) = 28.90. Open-field speed: F(2, 23) = 10.27. Open-field number of stops: F(2, 23) = 17.08. Sex did not modulate performance in R6/2 mice in open-field or rotarod tests. Data expressed as mean ± SEM. *p < 0.05 Tukey’s post hoc analysis after two-way ANOVA analysis (only genotype interactions were significant). n = 5 for male WT mice, 7 for female WT mice, 7 for male R6/2 mice, and 8 for female R6/2 mice. (AI 233 kb)
Supplementary Fig. 2
Study design. (AI 234 kb)
Supplementary Fig. 3
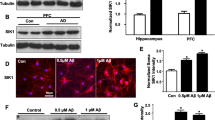
The effect of EGF treatment on behavior and HD-relevant pathology in wild-type mice. Wild-type (WT) mice were treated twice per week with vehicle (Veh) or EGF (300 μg/kg) from 4/5 to 10/11 weeks of age. a EGF treatment reduced rotarod performance but b did not modulate performance in open field. c EGF levels were higher in the plasma after EGF treatment. EGF treatment did not cause changes in d fibrinogen extravasation, e vessel coverage, or f levels of GABAergic proteins in the cortex. Data expressed as mean ± SEM. *p < 0.05 by Student’s t test. n = 5 per group. In (d), green, CD31; red, fibrinogen. In (e), green, laminin. Scale bar, 50 μm. (AI 1.11 kb)
Supplementary Fig. 4
EGF treatment does not modulate HD-relevant pathology in the striatum of R6/2 mice. Wild-type (WT) or R6/2 mice were treated twice per week with vehicle (Veh) or EGF (300 μg/kg) from 4/5 to 10/11 weeks of age. a Fibrinogen levels were higher in R6/2 mice compared to wild-type (WT) mice but were unaffected by EGF treatment in the striatum (IHC analysis). F(2, 16) = 77.23. b Cortical vessel coverage (laminin staining) was higher in R6/2 mice compared to vehicle-treated R6/2 mice but was also unaltered by EGF treatment in R6/2 mice. F(2, 16) = 3.997. c Top, representative images of fibrinogen (red) and brain endothelial cell (CD31, green) staining in the striatum. Bottom, representative images of laminin (green) staining in striatum. Scale bar, 50 μm. d PSD95 and DARPP-32 levels were lower in R6/2 mice compared to WT mice, whereas GAD67 and GAD65 levels were similar when assessed by western blot analysis. PSD95: F(2, 14) = 27.61. DARPP-32: F(2, 14) = 31. EGF treatment did not alter levels of PSD95, GAD65, GAD67, or DARPP-32 in the striatum of R6/2 mice. Left, quantification of each protein when normalized to KHC as a loading control. All data were then expressed as a ratio to vehicle-treated WT mice. Right, representative blots of each protein and loading control with bands on the same gel in nonadjacent positions separated by a dashed line. Data expressed as mean ± SEM. *p < 0.05 Tukey’s post hoc analysis after one-way ANOVA analysis. In (a)–(c), n = 5 for WT mice, 6 for vehicle-treated R6/2 mice, and 8 for EGF-treated R6/2 mice. In (d), n = 5 for WT mice, 6 for vehicle-treated R6/2 mice, and 6 for EGF-treated R6/2 mice. (AI 604 kb)
Supplementary Fig. 5
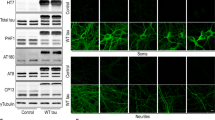
EGF treatment does not alter PSD95, NR1, or NR2A levels in the cortex of R6/2 mice. Wild-type (WT) or R6/2 mice were treated twice per week with vehicle (Veh) or EGF (300 μg/kg) from 4/5 to 10/11 weeks of age. Levels of PSD95, NR1, and NR2A were lower in R6/2 mice compared to WT. PSD95: F(2, 14) = 13.71. NR1: F(2, 14) = 23.84. NR2A: F(2, 14) = 38.23. However, EGF treatment did not alter levels of these markers in the cortex when assessed by western blot analysis. a Quantification of each protein when normalized to GAPDH as a loading control. All data were then expressed as a ratio to vehicle-treated WT mice. b Representative blots of each protein and loading control, with bands on the same gel in nonadjacent positions separated by a dashed line. Data expressed as mean ± SEM. *p < 0.05 using Tukey’s post hoc test after significant one-way ANOVA. n = 5 for WT mice, 6 for vehicle-treated R6/2 mice, and 6 for EGF-treated R6/2 mice. (AI 552 kb)
ESM 1
(DOCX 13.7 kb)
Rights and permissions
About this article
Cite this article
Marottoli, F.M., Priego, M., Flores-Barrera, E. et al. EGF Treatment Improves Motor Behavior and Cortical GABAergic Function in the R6/2 Mouse Model of Huntington’s Disease. Mol Neurobiol 56, 7708–7718 (2019). https://doi.org/10.1007/s12035-019-1634-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-019-1634-y