Abstract
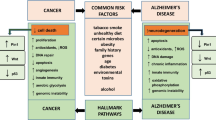
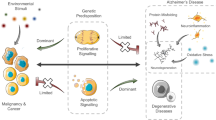
Although Alzheimer’s disease (AD) is an international health research priority for our aging population, little therapeutic progress has been made. This lack of progress may be partially attributable to disease heterogeneity. Previous studies have identified an inverse association of cancer and AD, suggesting that cancer history may be one source of AD heterogeneity. These findings are particularly interesting in light of the number of common risk factors and two-hit models hypothesized to commonly drive both diseases. We reviewed the ten hallmark biological alterations of cancer cells to investigate overlap with the AD literature and identified overlap of all ten hallmarks in AD, including (1) potentially common underlying risk factors, such as increased inflammation, deregulated cellular energetics, and genome instability; (2) inversely regulated mechanisms, including cell death and evading growth suppressors; and (3) functions with more complex, pleiotropic mechanisms, some of which may be stage-dependent in AD, such as cell adhesion/contact inhibition and angiogenesis. Additionally, we discuss the recent observation of a biological link between cancer and AD neuropathology. Finally, we address the therapeutic implications of this topic. The significant overlap of functional pathways and molecules between these diseases, some similarly and some oppositely regulated or functioning in each disease, supports the need for more research to elucidate cancer-related AD genetic and functional heterogeneity, with the aims of better understanding AD risk mediators, as well as further exploring the potential for some types of drug repurposing towards AD therapeutic development.


Similar content being viewed by others
References
Perl DP (2010) Neuropathology of Alzheimer’s disease. Mt Sinai J Med 77(1):32–42
Driver JA, Beiser A, Au R, Kreger BE, Splansky GL, Kurth T, Kiel DP, Lu KP et al (2012) Inverse association between cancer and Alzheimer’s disease: results from the Framingham Heart Study. BMJ 344:e1442
Musicco M, Adorni F, di Santo S, Prinelli F, Pettenati C, Caltagirone C, Palmer K, Russo A (2013) Inverse occurrence of cancer and Alzheimer disease: a population-based incidence study. Neurology 81(4):322–328
Nudelman KN et al (2014) Association of cancer history with Alzheimer’s disease onset and structural brain changes. Front Physiol 5:423
Roe CM, Behrens MI, Xiong C, Miller JP, Morris JC (2005) Alzheimer disease and cancer. Neurology 64(5):895–898
Roe CM, Fitzpatrick AL, Xiong C, Sieh W, Kuller L, Miller JP, Williams MM, Kopan R et al (2010) Cancer linked to Alzheimer disease but not vascular dementia. Neurology 74(2):106–112
Snyder HM, Ahles T, Calderwood S, Carrillo MC, Chen H, Chang CCH, Craft S, de Jager P et al (2017) Exploring the nexus of Alzheimer’s disease and related dementias with cancer and cancer therapies: a convening of the Alzheimer’s Association & Alzheimer’s Drug Discovery Foundation. Alzheimers Dement 13(3):267–273
Frain L, Swanson D, Cho K, Gagnon D, Lu KP, Betensky RA, Driver J (2017) Association of cancer and Alzheimer’s disease risk in a national cohort of veterans. Alzheimers Dement 13:1364–1370
Li JQ, Tan L, Wang HF, Tan MS, Tan L, Xu W, Zhao QF, Wang J et al (2016) Risk factors for predicting progression from mild cognitive impairment to Alzheimer’s disease: a systematic review and meta-analysis of cohort studies. J Neurol Neurosurg Psychiatry 87(5):476–484
Vanhanen M, Koivisto K, Moilanen L, Helkala EL, Hanninen T, Soininen H, Kervinen K, Kesaniemi YA et al (2006) Association of metabolic syndrome with Alzheimer disease: a population-based study. Neurology 67(5):843–847
Roskies M, Dolev Y, Caglar D, Hier MP, Mlynarek A, Majdan A, Payne RJ (2012) Vitamin D deficiency as a potentially modifiable risk factor for thyroid cancer. J Otolaryngol Head Neck Surg 41(3):160–163
Malekshah AF, Kimiagar M, Pourshams A, Yazdani J, Kaiedi Majd S, Goglani G, Jaafari E, Semnani S et al (2010) Vitamin deficiency in Golestan Province, northern Iran: a high-risk area for esophageal cancer. Arch Iran Med 13(5):391–394
Baena Ruiz, R. and P. Salinas Hernandez, Diet and cancer: risk factors and epidemiological evidence. Maturitas, 2014. 77(3): p. 202–208.
Palmer S (1985) Diet, nutrition, and cancer. Prog Food Nutr Sci 9(3–4):283–341
Mosconi L, McHugh PF (2015) Let food be thy medicine: diet, nutrition, and biomarkers’ risk of Alzheimer’s disease. Curr Nutr Rep 4(2):126–135
Hoel DG, Berwick M, de Gruijl FR, Holick MF (2016) The risks and benefits of sun exposure 2016. Dermatoendocrinol 8(1):e1248325
Mayeux, R. and Y. Stern, Epidemiology of Alzheimer disease. Cold Spring Harb Perspect Med, 2012. 2(8).
Zhong G, Wang Y, Zhang Y, Guo JJ, Zhao Y (2015) Smoking is associated with an increased risk of dementia: a meta-analysis of prospective cohort studies with investigation of potential effect modifiers. PLoS One 10(3):e0118333
Ordonez-Mena JM et al (2016) Quantification of the smoking-associated cancer risk with rate advancement periods: meta-analysis of individual participant data from cohorts of the CHANCES consortium. BMC Med 14:62
Fair AM, Montgomery K (2009) Energy balance, physical activity, and cancer risk. Methods Mol Biol 472:57–88
Barnard RJ, Aronson WJ (2005) Preclinical models relevant to diet, exercise, and cancer risk. Recent Results Cancer Res 166:47–61
Mates JM et al (2010) Roles of dioxins and heavy metals in cancer and neurological diseases using ROS-mediated mechanisms. Free Radic Biol Med 49(9):1328–1341
Wu J, Basha MR, Brock B, Cox DP, Cardozo-Pelaez F, McPherson CA, Harry J, Rice DC et al (2008) Alzheimer’s disease (AD)-like pathology in aged monkeys after infantile exposure to environmental metal lead (Pb): evidence for a developmental origin and environmental link for AD. J Neurosci 28(1):3–9
Nunez O et al (2017) Association between heavy metal and metalloid levels in topsoil and cancer mortality in Spain. Environ Sci Pollut Res Int
Holohan KN et al (2012) Functional microRNAs in Alzheimer’s disease and cancer: differential regulation of common mechanisms and pathways. Front Genet 3:323
Satoh J (2012) Molecular network analysis of human microRNA targetome: from cancers to Alzheimer’s disease. BioData Min 5(1):17
Ibanez K et al (2014) Molecular evidence for the inverse comorbidity between central nervous system disorders and cancers detected by transcriptomic meta-analyses. PLoS Genet 10(2):–e1004173
Knudson AG Jr (1971) Mutation and cancer: statistical study of retinoblastoma. Proc Natl Acad Sci U S A 68(4):820–823
Lahiri DK, Maloney B (2010) The “LEARn” (Latent Early-life Associated Regulation) model integrates environmental risk factors and the developmental basis of Alzheimer’s disease, and proposes remedial steps. Exp Gerontol 45(4):291–296
Lahiri DK et al (2007) How and when environmental agents and dietary factors affect the course of Alzheimer’s disease: the “LEARn” model (latent early-life associated regulation) may explain the triggering of AD. Curr Alzheimer Res 4(2):219–228
Lahiri DK, Maloney B, Zawia NH (2009) The LEARn model: an epigenetic explanation for idiopathic neurobiological diseases. Mol Psychiatry 14(11):992–1003
Lahiri DK, Zawia NH, Greig NH, Sambamurti K, Maloney B (2008) Early-life events may trigger biochemical pathways for Alzheimer’s disease: the “LEARn” model. Biogerontology 9(6):375–379
Maloney B, Sambamurti K, Zawia N, K. Lahiri D (2012) Applying epigenetics to Alzheimer’s disease via the latent early-life associated regulation (LEARn) model. Curr Alzheimer Res 9(5):589–599
Hanahan D, Weinberg RA (2000) The hallmarks of cancer. Cell 100(1):57–70
Hanahan D, Weinberg RA (2011) Hallmarks of cancer: the next generation. Cell 144(5):646–674
Leroy B, Fournier JL, Ishioka C, Monti P, Inga A, Fronza G, Soussi T (2013) The TP53 website: an integrative resource centre for the TP53 mutation database and TP53 mutant analysis. Nucleic Acids Res 41(Database issue):D962–D969
Petitjean A, Mathe E, Kato S, Ishioka C, Tavtigian SV, Hainaut P, Olivier M (2007) Impact of mutant p53 functional properties on TP53 mutation patterns and tumor phenotype: lessons from recent developments in the IARC TP53 database. Hum Mutat 28(6):622–629
Olivier M, Goldgar DE, Sodha N, Ohgaki H, Kleihues P, Hainaut P, Eeles RA (2003) Li-Fraumeni and related syndromes: correlation between tumor type, family structure, and TP53 genotype. Cancer Res 63(20):6643–6650
Tur, M.K., et al., Restoration of DAP kinase tumor suppressor function: a therapeutic strategy to selectively induce apoptosis in cancer cells using immunokinase fusion proteins. Biomedicines, 2017. 5(4).
LeBlanc AC (2005) The role of apoptotic pathways in Alzheimer’s disease neurodegeneration and cell death. Curr Alzheimer Res 2(4):389–402
Zhu X, Raina A, Perry G, Smith M (2006) Apoptosis in Alzheimer disease: a mathematical improbability. Curr Alzheimer Res 3(4):393–396
Hamdane M, Delobel P, Sambo AV, Smet C, Bégard S, Violleau A, Landrieu I, Delacourte A et al (2003) Neurofibrillary degeneration of the Alzheimer-type: an alternate pathway to neuronal apoptosis? Biochem Pharmacol 66(8):1619–1625
Zhao S, Zhao J, Zhang T, Guo C (2016) Increased apoptosis in the platelets of patients with Alzheimer’s disease and amnestic mild cognitive impairment. Clin Neurol Neurosurg 143:46–50
Czech C, Tremp G, Pradier L (2000) Presenilins and Alzheimer’s disease: biological functions and pathogenic mechanisms. Prog Neurobiol 60(4):363–384
Kovacs DM et al (1999) Staurosporine-induced activation of caspase-3 is potentiated by presenilin 1 familial Alzheimer’s disease mutations in human neuroglioma cells. J Neurochem 73(6):2278–2285
Akhter R, Sanphui P, Biswas SC (2014) The essential role of p53-up-regulated modulator of apoptosis (Puma) and its regulation by FoxO3a transcription factor in beta-amyloid-induced neuron death. J Biol Chem 289(15):10812–10822
Akhter R, Sanphui P, Das H, Saha P, Biswas SC (2015) The regulation of p53 up-regulated modulator of apoptosis by JNK/c-Jun pathway in beta-amyloid-induced neuron death. J Neurochem 134(6):1091–1103
Yu W, Mechawar N, Krantic S, Quirion R (2010) Evidence for the involvement of apoptosis-inducing factor-mediated caspase-independent neuronal death in Alzheimer disease. Am J Pathol 176(5):2209–2218
You MH, Kim BM, Chen CH, Begley MJ, Cantley LC, Lee TH (2017) Death-associated protein kinase 1 phosphorylates NDRG2 and induces neuronal cell death. Cell Death Differ 24(2):238–250
Cantwell-Dorris ER, O’Leary JJ, Sheils OM (2011) BRAFV600E: implications for carcinogenesis and molecular therapy. Mol Cancer Ther 10(3):385–394
Bos JL (1989) ras oncogenes in human cancer: a review. Cancer Res 49(17):4682–4689
Santarpia L, Lippman SM, El-Naggar AK (2012) Targeting the MAPK-RAS-RAF signaling pathway in cancer therapy. Expert Opin Ther Targets 16(1):103–119
Nelson DM, McBryan T, Jeyapalan JC, Sedivy JM, Adams PD (2014) A comparison of oncogene-induced senescence and replicative senescence: implications for tumor suppression and aging. Age (Dordr) 36(3):9637
Ferrer I et al (2001) Phosphorylated mitogen-activated protein kinase (MAPK/ERK-P), protein kinase of 38 kDa (p38-P), stress-activated protein kinase (SAPK/JNK-P), and calcium/calmodulin-dependent kinase II (CaM kinase II) are differentially expressed in tau deposits in neurons and glial cells in tauopathies. J Neural Transm (Vienna) 108(12):1397–1415
Cavallini A, Brewerton S, Bell A, Sargent S, Glover S, Hardy C, Moore R, Calley J et al (2013) An unbiased approach to identifying tau kinases that phosphorylate tau at sites associated with Alzheimer disease. J Biol Chem 288(32):23331–23347
Gartner U et al (1995) Induction of p21ras in Alzheimer pathology. Neuroreport 6(10):1441–1444
Gartner U, Holzer M, Arendt T (1999) Elevated expression of p21ras is an early event in Alzheimer’s disease and precedes neurofibrillary degeneration. Neuroscience 91(1):1–5
Hallock P, Thomas MA (2012) Integrating the Alzheimer’s disease proteome and transcriptome: a comprehensive network model of a complex disease. OMICS 16(1–2):37–49
Liu T, Ren D, Zhu X, Yin Z, Jin G, Zhao Z, Robinson D, Li X et al (2013) Transcriptional signaling pathways inversely regulated in Alzheimer’s disease and glioblastoma multiform. Sci Rep 3:3467
Liang WS, Dunckley T, Beach TG, Grover A, Mastroeni D, Ramsey K, Caselli RJ, Kukull WA et al (2008) Altered neuronal gene expression in brain regions differentially affected by Alzheimer’s disease: a reference data set. Physiol Genomics 33(2):240–256
Ferrer I, Gomez-Isla T, Puig B, Freixes M, Ribe E, Dalfo E, Avila J (2005) Current advances on different kinases involved in tau phosphorylation, and implications in Alzheimer’s disease and tauopathies. Curr Alzheimer Res 2(1):3–18
Zhu X, Raina AK, Rottkamp CA, Aliev G, Perry G, Boux H, Smith MA (2001) Activation and redistribution of c-jun N-terminal kinase/stress activated protein kinase in degenerating neurons in Alzheimer’s disease. J Neurochem 76(2):435–441
Griffin RJ, Moloney A, Kelliher M, Johnston JA, Ravid R, Dockery P, O’Connor R, O’Neill C (2005) Activation of Akt/PKB, increased phosphorylation of Akt substrates and loss and altered distribution of Akt and PTEN are features of Alzheimer’s disease pathology. J Neurochem 93(1):105–117
Robinson DR, Wu YM, Lonigro RJ, Vats P, Cobain E, Everett J, Cao X, Rabban E et al (2017) Integrative clinical genomics of metastatic cancer. Nature 548:297–303
Fischer M, Grossmann P, Padi M, DeCaprio JA (2016) Integration of TP53, DREAM, MMB-FOXM1 and RB-E2F target gene analyses identifies cell cycle gene regulatory networks. Nucleic Acids Res 44(13):6070–6086
Lim HJ, Crowe P, Yang JL (2015) Current clinical regulation of PI3K/PTEN/Akt/mTOR signalling in treatment of human cancer. J Cancer Res Clin Oncol 141(4):671–689
Wall M, Poortinga G, Hannan KM, Pearson RB, Hannan RD, McArthur GA (2008) Translational control of c-MYC by rapamycin promotes terminal myeloid differentiation. Blood 112(6):2305–2317
Keeney JT et al (2012) Cell cycle proteins in brain in mild cognitive impairment: insights into progression to Alzheimer disease. Neurotox Res 22(3):220–230
Bonda DJ, Lee HP, Kudo W, Zhu X, Smith MA, Lee HG (2010) Pathological implications of cell cycle re-entry in Alzheimer disease. Expert Rev Mol Med 12:e19
Khurana V, Lu Y, Steinhilb ML, Oldham S, Shulman JM, Feany MB (2006) TOR-mediated cell-cycle activation causes neurodegeneration in a Drosophila tauopathy model. Curr Biol 16(3):230–241
Absalon S, Kochanek DM, Raghavan V, Krichevsky AM (2013) MiR-26b, upregulated in Alzheimer’s disease, activates cell cycle entry, tau-phosphorylation, and apoptosis in postmitotic neurons. J Neurosci 33(37):14645–14659
Lee HG, Casadesus G, Zhu X, Castellani RJ, McShea A, Perry G, Petersen RB, Bajic V et al (2009) Cell cycle re-entry mediated neurodegeneration and its treatment role in the pathogenesis of Alzheimer’s disease. Neurochem Int 54(2):84–88
Blalock EM, Geddes JW, Chen KC, Porter NM, Markesbery WR, Landfield PW (2004) Incipient Alzheimer’s disease: microarray correlation analyses reveal major transcriptional and tumor suppressor responses. Proc Natl Acad Sci U S A 101(7):2173–2178
Sonoda Y, Mukai H, Matsuo K, Takahashi M, Ono Y, Maeda K, Akiyama H, Kawamata T (2010) Accumulation of tumor-suppressor PTEN in Alzheimer neurofibrillary tangles. Neurosci Lett 471(1):20–24
Wilson C, Henry S, Smith MA, Bowser R (2004) The p53 homologue p73 accumulates in the nucleus and localizes to neurites and neurofibrillary tangles in Alzheimer disease brain. Neuropathol Appl Neurobiol 30(1):19–29
Arendt T, Rödel L, Gärtner U, Holzer M (1996) Expression of the cyclin-dependent kinase inhibitor p16 in Alzheimer’s disease. Neuroreport 7(18):3047–3049
Lovell MA, Xie C, Xiong S, Markesbery WR (2003) Wilms’ tumor suppressor (WT1) is a mediator of neuronal degeneration associated with the pathogenesis of Alzheimer’s disease. Brain Res 983(1–2):84–96
Munoz U et al (2008) Enhanced proteasome-dependent degradation of the CDK inhibitor p27(kip1) in immortalized lymphocytes from Alzheimer’s dementia patients. Neurobiol Aging 29(10):1474–1484
Ogawa O, Lee HG, Zhu X, Raina A, Harris PLR, Castellani RJ, Perry G, Smith MA (2003) Increased p27, an essential component of cell cycle control, in Alzheimer’s disease. Aging Cell 2(2):105–110
Kim H, Kwon YA, Ahn IS, Kim S, Kim S, Jo SA, Kim DK (2016) Overexpression of cell cycle proteins of peripheral lymphocytes in patients with Alzheimer’s disease. Psychiatry Investig 13(1):127–134
Song J, Wang S, Tan M, Jia J (2012) G1/S checkpoint proteins in peripheral blood lymphocytes are potentially diagnostic biomarkers for Alzheimer’s disease. Neurosci Lett 526(2):144–149
Tan M, Wang S, Song J, Jia J (2012) Combination of p53(ser15) and p21/p21(thr145) in peripheral blood lymphocytes as potential Alzheimer’s disease biomarkers. Neurosci Lett 516(2):226–231
Hayflick, L., Mortality and immortality at the cellular level. A review. Biochemistry (Mosc), 1997. 62(11): p. 1180–1190.
Bryan TM, Cech TR (1999) Telomerase and the maintenance of chromosome ends. Curr Opin Cell Biol 11(3):318–324
Counter CM, Avilion AA, LeFeuvre CE, Stewart NG, Greider CW, Harley CB, Bacchetti S (1992) Telomere shortening associated with chromosome instability is arrested in immortal cells which express telomerase activity. EMBO J 11(5):1921–1929
Counter CM, Hahn WC, Wei W, Caddle SD, Beijersbergen RL, Lansdorp PM, Sedivy JM, Weinberg RA (1998) Dissociation among in vitro telomerase activity, telomere maintenance, and cellular immortalization. Proc Natl Acad Sci U S A 95(25):14723–14728
Akincilar SC, Unal B, Tergaonkar V (2016) Reactivation of telomerase in cancer. Cell Mol Life Sci 73(8):1659–1670
Forero DA, González-Giraldo Y, López-Quintero C, Castro-Vega LJ, Barreto GE, Perry G (2016) Meta-analysis of telomere length in Alzheimer’s disease. J Gerontol A Biol Sci Med Sci 71(8):1069–1073
Zhan Y, Song C, Karlsson R, Tillander A, Reynolds CA, Pedersen NL, Hägg S (2015) Telomere length shortening and Alzheimer disease--a Mendelian randomization study. JAMA Neurol 72(10):1202–1203
Horgusluoglu E, Nudelman K, Nho K, Saykin AJ (2017) Adult neurogenesis and neurodegenerative diseases: a systems biology perspective. Am J Med Genet B Neuropsychiatr Genet 174(1):93–112
Mamdani F, Rollins B, Morgan L, Myers RM, Barchas JD, Schatzberg AF, Watson SJ, Akil H et al (2015) Variable telomere length across post-mortem human brain regions and specific reduction in the hippocampus of major depressive disorder. Transl Psychiatry 5:e636
Wolkowitz OM, Mellon SH, Lindqvist D, Epel ES, Blackburn EH, Lin J, Reus VI, Burke H et al (2015) PBMC telomerase activity, but not leukocyte telomere length, correlates with hippocampal volume in major depression. Psychiatry Res 232(1):58–64
Yun S, Donovan MH, Ross MN, Richardson DR, Reister R, Farnbauch LA, Fischer SJ, Riethmacher D et al (2016) Stress-induced anxiety- and depressive-like phenotype associated with transient reduction in neurogenesis in adult nestin-CreERT2/diphtheria toxin fragment a transgenic mice. PLoS One 11(1):e0147256
Gonzalez-Giraldo Y et al (2016) Neuroprotective effects of the catalytic subunit of telomerase: a potential therapeutic target in the central nervous system. Ageing Res Rev 28:37–45
Folkman J (1971) Tumor angiogenesis: therapeutic implications. N Engl J Med 285(21):1182–1186
Otrock ZK et al (2007) Understanding the biology of angiogenesis: review of the most important molecular mechanisms. Blood Cells Mol Dis 39(2):212–220
Wierenga CE, Hays CC, Zlatar ZZ (2014) Cerebral blood flow measured by arterial spin labeling MRI as a preclinical marker of Alzheimer’s disease. J Alzheimers Dis 42(Suppl 4):S411–S419
Tosun D, Schuff N, Jagust W, Weiner MW (2016) Discriminative power of arterial spin labeling magnetic resonance imaging and 18F-fluorodeoxyglucose positron emission tomography changes for amyloid-beta-positive subjects in the Alzheimer’s disease continuum. Neurodegener Dis 16(1–2):87–94
Okonkwo OC, Xu G, Oh JM, Dowling NM, Carlsson CM, Gallagher CL, Birdsill AC, Palotti M et al (2014) Cerebral blood flow is diminished in asymptomatic middle-aged adults with maternal history of Alzheimer’s disease. Cereb Cortex 24(4):978–988
Franceschi M, Alberoni M, Bressi S, Canal N, Comi G, Fazio F, Grassi F, Perani D et al (1995) Correlations between cognitive impairment, middle cerebral artery flow velocity and cortical glucose metabolism in the early phase of Alzheimer’s disease. Dementia 6(1):32–38
Tohgi H, Yonezawa H, Takahashi S, Sato N, Kato E, Kudo M, Hatano K, Sasaki T (1998) Cerebral blood flow and oxygen metabolism in senile dementia of Alzheimer’s type and vascular dementia with deep white matter changes. Neuroradiology 40(3):131–137
Schneider JA, Wilson RS, Bienias JL, Evans DA, Bennett DA (2004) Cerebral infarctions and the likelihood of dementia from Alzheimer disease pathology. Neurology 62(7):1148–1155
Schneider JA, Boyle PA, Arvanitakis Z, Bienias JL, Bennett DA (2007) Subcortical infarcts, Alzheimer’s disease pathology, and memory function in older persons. Ann Neurol 62(1):59–66
Schneider JA, Arvanitakis Z, Leurgans SE, Bennett DA (2009) The neuropathology of probable Alzheimer disease and mild cognitive impairment. Ann Neurol 66(2):200–208
Thal DR, Griffin WST, de Vos RAI, Ghebremedhin E (2008) Cerebral amyloid angiopathy and its relationship to Alzheimer’s disease. Acta Neuropathol 115(6):599–609
Jeynes B, Provias J (2006) The possible role of capillary cerebral amyloid angiopathy in Alzheimer lesion development: a regional comparison. Acta Neuropathol 112(4):417–427
Ashok BS, Ajith TA, Sivanesan S (2017) Hypoxia-inducible factors as neuroprotective agent in Alzheimer’s disease. Clin Exp Pharmacol Physiol 44(3):327–334
Salminen A, Kauppinen A, Kaarniranta K (2017) Hypoxia/ischemia activate processing of amyloid precursor protein: impact of vascular dysfunction in the pathogenesis of Alzheimer's disease. J Neurochem 140(4):536–549
Provias J, Jeynes B (2008) Neurofibrillary tangles and senile plaques in Alzheimer’s brains are associated with reduced capillary expression of vascular endothelial growth factor and endothelial nitric oxide synthase. Curr Neurovasc Res 5(3):199–205
Castellano E, Molina-Arcas M, Krygowska AA, East P, Warne P, Nicol A, Downward J (2016) RAS signalling through PI3-Kinase controls cell migration via modulation of Reelin expression. Nat Commun 7:11245
Angelucci C, Maulucci G, Lama G, Proietti G, Colabianchi A, Papi M, Maiorana A, de Spirito M et al (2012) Epithelial-stromal interactions in human breast cancer: effects on adhesion, plasma membrane fluidity and migration speed and directness. PLoS One 7(12):e50804
Ramanan VK et al (2012) Genome-wide pathway analysis of memory impairment in the Alzheimer’s Disease Neuroimaging Initiative (ADNI) cohort implicates gene candidates, canonical pathways, and networks. Brain Imaging Behav 6(4):634–648
Liu G, Jiang Y, Wang P, Feng R, Jiang N, Chen X, Song H, Chen Z (2012) Cell adhesion molecules contribute to Alzheimer’s disease: multiple pathway analyses of two genome-wide association studies. J Neurochem 120(1):190–198
Leshchyns'ka I, Sytnyk V (2016) Synaptic cell adhesion molecules in Alzheimer’s disease. Neural Plast 2016:6427537
Chin J, Massaro CM, Palop JJ, Thwin MT, Yu GQ, Bien-Ly N, Bender A, Mucke L (2007) Reelin depletion in the entorhinal cortex of human amyloid precursor protein transgenic mice and humans with Alzheimer’s disease. J Neurosci 27(11):2727–2733
Kocherhans S, Madhusudan A, Doehner J, Breu KS, Nitsch RM, Fritschy JM, Knuesel I (2010) Reduced Reelin expression accelerates amyloid-beta plaque formation and tau pathology in transgenic Alzheimer’s disease mice. J Neurosci 30(27):9228–9240
Deutsch SI, Rosse RB, Deutsch LH (2006) Faulty regulation of tau phosphorylation by the reelin signal transduction pathway is a potential mechanism of pathogenesis and therapeutic target in Alzheimer’s disease. Eur Neuropsychopharmacol 16(8):547–551
Knuesel I, Nyffeler M, Mormède C, Muhia M, Meyer U, Pietropaolo S, Yee BK, Pryce CR et al (2009) Age-related accumulation of Reelin in amyloid-like deposits. Neurobiol Aging 30(5):697–716
Cuchillo-Ibanez I et al (2016) The beta-amyloid peptide compromises Reelin signaling in Alzheimer’s disease. Sci Rep 6:31646
Konishi H, Mohseni M, Tamaki A, Garay JP, Croessmann S, Karnan S, Ota A, Wong HY et al (2011) Mutation of a single allele of the cancer susceptibility gene BRCA1 leads to genomic instability in human breast epithelial cells. Proc Natl Acad Sci U S A 108(43):17773–17778
Desmaze C, Soria JC, Freulet-Marrière MA, Mathieu N, Sabatier L (2003) Telomere-driven genomic instability in cancer cells. Cancer Lett 194(2):173–182
Forsberg LA, Rasi C, Malmqvist N, Davies H, Pasupulati S, Pakalapati G, Sandgren J, de Ståhl TD et al (2014) Mosaic loss of chromosome Y in peripheral blood is associated with shorter survival and higher risk of cancer. Nat Genet 46(6):624–628
Noveski P, Madjunkova S, Sukarova Stefanovska E, Matevska Geshkovska N, Kuzmanovska M, Dimovski A, Plaseska-Karanfilska D (2016) Loss of Y chromosome in peripheral blood of colorectal and prostate Cancer patients. PLoS One 11(1):e0146264
Wright DJ, Day FR, Kerrison ND, Zink F, Cardona A, Sulem P, Thompson DJ, Sigurjonsdottir S et al (2017) Genetic variants associated with mosaic Y chromosome loss highlight cell cycle genes and overlap with cancer susceptibility. Nat Genet 49(5):674–679
Minner S, Kilgué A, Stahl P, Weikert S, Rink M, Dahlem R, Fisch M, Höppner W et al (2010) Y chromosome loss is a frequent early event in urothelial bladder cancer. Pathology 42(4):356–359
Yurov YB, Vorsanova SG, Iourov IY (2011) The DNA replication stress hypothesis of Alzheimer’s disease. ScientificWorldJournal 11:2602–2612
Arendt T, Brückner MK, Mosch B, Lösche A (2010) Selective cell death of hyperploid neurons in Alzheimer’s disease. Am J Pathol 177(1):15–20
Arendt T (2012) Cell cycle activation and aneuploid neurons in Alzheimer’s disease. Mol Neurobiol 46(1):125–135
Yang Y, Geldmacher DS, Herrup K (2001) DNA replication precedes neuronal cell death in Alzheimer’s disease. J Neurosci 21(8):2661–2668
Mosch B, Morawski M, Mittag A, Lenz D, Tarnok A, Arendt T (2007) Aneuploidy and DNA replication in the normal human brain and Alzheimer’s disease. J Neurosci 27(26):6859–6867
van den Bos H, Spierings DC, Taudt AS, Bakker B, Porubský D, Falconer E, Novoa C, Halsema N et al (2016) Single-cell whole genome sequencing reveals no evidence for common aneuploidy in normal and Alzheimer’s disease neurons. Genome Biol 17(1):116
Spremo-Potparevic B, Bajic V, Perry G, Zivkovic L (2015) Alterations of the X chromosome in lymphocytes of Alzheimer’s disease patients. Curr Alzheimer Res 12(10):990–996
Dumanski JP, Lambert JC, Rasi C, Giedraitis V, Davies H, Grenier-Boley B, Lindgren CM, Campion D et al (2016) Mosaic loss of chromosome Y in blood is associated with Alzheimer disease. Am J Hum Genet 98(6):1208–1219
Spremo-Potparevic B et al (2008) Premature centromere division of the X chromosome in neurons in Alzheimer’s disease. J Neurochem 106(5):2218–2223
Migliore L, Coppede F, Fenech M, Thomas P (2011) Association of micronucleus frequency with neurodegenerative diseases. Mutagenesis 26(1):85–92
Liu M, Huo YR, Wang J, Wang C, Liu S, Liu S, Wang J, Ji Y (2016) Telomere shortening in Alzheimer’s disease patients. Ann Clin Lab Sci 46(3):260–265
Mathur S, Glogowska A, McAvoy E, Righolt C, Rutherford J, Willing C, Banik U, Ruthirakuhan M et al (2014) Three-dimensional quantitative imaging of telomeres in buccal cells identifies mild, moderate, and severe Alzheimer’s disease patients. J Alzheimers Dis 39(1):35–48
Lukens JN, van Deerlin V, Clark CM, Xie SX, Johnson FB (2009) Comparisons of telomere lengths in peripheral blood and cerebellum in Alzheimer’s disease. Alzheimers Dement 5(6):463–469
Thomas, P., O.C. NJ, and M. Fenech, Telomere length in white blood cells, buccal cells and brain tissue and its variation with ageing and Alzheimer’s disease. Mech Ageing Dev, 2008. 129(4): p. 183–190.
Guan JZ, Guan WP, Maeda T, Makino N (2013) Analysis of telomere length and subtelomeric methylation of circulating leukocytes in women with Alzheimer’s disease. Aging Clin Exp Res 25(1):17–23
Roberts RO, Boardman LA, Cha RH, Pankratz VS, Johnson RA, Druliner BR, Christianson TJH, Roberts LR et al (2014) Short and long telomeres increase risk of amnestic mild cognitive impairment. Mech Ageing Dev 141–142:64–69
Singh K, Singh K (2015) Carcinogenesis and diabetic wound healing: evidences of parallelism. Curr Diabetes Rev 11(1):32–45
Goswami KK, Ghosh T, Ghosh S, Sarkar M, Bose A, Baral R (2017) Tumor promoting role of anti-tumor macrophages in tumor microenvironment. Cell Immunol 316:1–10
van de Nieuwenhof HP, Hebeda KM, Bulten J, Otte-Holler I, Massuger LFAG, de Hullu JA, van Kempen LCLT (2010) Specific intraepithelial localization of mast cells in differentiated vulvar intraepithelial neoplasia and its possible contribution to vulvar squamous cell carcinoma development. Histopathology 57(3):351–362
Tang X, Wang S, An C, du P, Yang Y (2017) Preoperative high neutrophil-to-lymphocyte ratio is associated with high-grade bladder cancer. Anticancer Res 37(8):4659–4663
Lieto E, Galizia G, Auricchio A, Cardella F, Mabilia A, Basile N, del Sorbo G, Castellano P et al (2017) Preoperative neutrophil to lymphocyte ratio and lymphocyte to monocyte ratio are prognostic factors in gastric cancers undergoing surgery. J Gastrointest Surg 21:1764–1774
DeNardo DG, Andreu P, Coussens LM (2010) Interactions between lymphocytes and myeloid cells regulate pro- versus anti-tumor immunity. Cancer Metastasis Rev 29(2):309–316
McGeer PL, McGeer EG (1998) Glial cell reactions in neurodegenerative diseases: pathophysiology and therapeutic interventions. Alzheimer Dis Assoc Disord 12(Suppl 2):S1–S6
McGeer PL, McGeer EG (1995) The inflammatory response system of brain: implications for therapy of Alzheimer and other neurodegenerative diseases. Brain Res Brain Res Rev 21(2):195–218
Serpente M, Bonsi R, Scarpini E, Galimberti D (2014) Innate immune system and inflammation in Alzheimer’s disease: from pathogenesis to treatment. Neuroimmunomodulation 21(2–3):79–87
Guillot-Sestier MV, Town T (2013) Innate immunity in Alzheimer’s disease: a complex affair. CNS Neurol Disord Drug Targets 12(5):593–607
Heneka MT, Golenbock DT, Latz E (2015) Innate immunity in Alzheimer’s disease. Nat Immunol 16(3):229–236
De Felice FG, Ferreira ST (2014) Inflammation, defective insulin signaling, and mitochondrial dysfunction as common molecular denominators connecting type 2 diabetes to Alzheimer disease. Diabetes 63(7):2262–2272
De la Fuente M, Miquel J (2009) An update of the oxidation-inflammation theory of aging: the involvement of the immune system in oxi-inflamm-aging. Curr Pharm Des 15(26):3003–3026
Warburg O (1925) The metabolism of carcinoma cells. Journal of Cancer Research 9(1):148–163
Xu XD, Shao SX, Jiang HP, Cao YW, Wang YH, Yang XC, Wang YL, Wang XS et al (2015) Warburg effect or reverse Warburg effect? A review of cancer metabolism. Oncol Res Treat 38(3):117–122
Pavlides S, Tsirigos A, Vera I, Flomenberg N, Frank PG, Casimiro MC, Wang C, Fortina P et al (2010) Loss of stromal caveolin-1 leads to oxidative stress, mimics hypoxia and drives inflammation in the tumor microenvironment, conferring the “reverse Warburg effect”: a transcriptional informatics analysis with validation. Cell Cycle 9(11):2201–2219
Pavlides S, Tsirigos A, Vera I, Flomenberg N, Frank PG, Casimiro MC, Wang C, Pestell RG et al (2010) Transcriptional evidence for the “reverse Warburg effect” in human breast cancer tumor stroma and metastasis: similarities with oxidative stress, inflammation, Alzheimer’s disease, and “neuron-glia metabolic coupling”. Aging (Albany NY) 2(4):185–199
Pavlides S, Whitaker-Menezes D, Castello-Cros R, Flomenberg N, Witkiewicz AK, Frank PG, Casimiro MC, Wang C et al (2009) The reverse Warburg effect: aerobic glycolysis in cancer associated fibroblasts and the tumor stroma. Cell Cycle 8(23):3984–4001
de Leon MJ, George AE, Ferris SH, Rosenbloom S, Christman DR, Gentes CI, Reisberg B, Kricheff II et al (1983) Regional correlation of PET and CT in senile dementia of the Alzheimer type. AJNR Am J Neuroradiol 4(3):553–556
de la Monte SM, Wands JR (2008) Alzheimer’s disease is type 3 diabetes-evidence reviewed. J Diabetes Sci Technol 2(6):1101–1113
Steen E, Terry BM, J. Rivera E, Cannon JL, Neely TR, Tavares R, Xu XJ, Wands JR et al (2005) Impaired insulin and insulin-like growth factor expression and signaling mechanisms in Alzheimer’s disease--is this type 3 diabetes? J Alzheimers Dis 7(1):63–80
de Leon MJ, Ferris SH, George AE, Christman DR, Fowler JS, Gentes C, Reisberg B, Gee B et al (1983) Positron emission tomographic studies of aging and Alzheimer disease. AJNR Am J Neuroradiol 4(3):568–571
Cutler NR (1986) Cerebral metabolism as measured with positron emission tomography (PET) and [18F] 2-deoxy-D-glucose: healthy aging, Alzheimer’s disease and Down syndrome. Prog Neuro-Psychopharmacol Biol Psychiatry 10(3–5):309–321
McGeer PL, McGeer EG, Hisaki K, Wong K (1986) Positron emission tomography and the possible origins of cytopathology in Alzheimer’s disease. Prog Neuro-Psychopharmacol Biol Psychiatry 10(3–5):501–518
Tamminga CA, Foster NL, Fedio P, Bird ED, Chase TN (1987) Alzheimer’s disease: low cerebral somatostatin levels correlate with impaired cognitive function and cortical metabolism. Neurology 37(1):161–165
Mann UM, Mohr E, Gearing M, Chase TN (1992) Heterogeneity in Alzheimer’s disease: progression rate segregated by distinct neuropsychological and cerebral metabolic profiles. J Neurol Neurosurg Psychiatry 55(10):956–959
Silverman DH et al (2001) Positron emission tomography in evaluation of dementia: regional brain metabolism and long-term outcome. JAMA 286(17):2120–2127
Alexander GE, Chen K, Pietrini P, Rapoport SI, Reiman EM (2002) Longitudinal PET evaluation of cerebral metabolic decline in dementia: a potential outcome measure in Alzheimer’s disease treatment studies. Am J Psychiatry 159(5):738–745
Ding F, Yao J, Rettberg JR, Chen S, Brinton RD (2013) Early decline in glucose transport and metabolism precedes shift to ketogenic system in female aging and Alzheimer’s mouse brain: implication for bioenergetic intervention. PLoS One 8(11):e79977
Henderson ST (2008) Ketone bodies as a therapeutic for Alzheimer’s disease. Neurotherapeutics 5(3):470–480
Hertz L, Chen Y, Waagepetersen HS (2015) Effects of ketone bodies in Alzheimer’s disease in relation to neural hypometabolism, beta-amyloid toxicity, and astrocyte function. J Neurochem 134(1):7–20
VanItallie TB (2015) Biomarkers, ketone bodies, and the prevention of Alzheimer’s disease. Metabolism 64(3 Suppl 1):S51–S57
Cunnane SC et al (2016) Can ketones help rescue brain fuel supply in later life? Implications for cognitive health during aging and the treatment of Alzheimer’s disease. Front Mol Neurosci 9:53
Small SA, Duff K (2008) Linking Abeta and tau in late-onset Alzheimer’s disease: a dual pathway hypothesis. Neuron 60(4):534–542
Osborn JL, Greer SF (2015) Metastatic melanoma cells evade immune detection by silencing STAT1. Int J Mol Sci 16(2):4343–4361
Pandey JP (2014) Immunoglobulin GM genes, cytomegalovirus immunoevasion, and the risk of glioma, neuroblastoma, and breast cancer. Front Oncol 4:236
Kawasaki BT, Farrar WL (2008) Cancer stem cells, CD200 and immunoevasion. Trends Immunol 29(10):464–468
McManus RM, Mills KH, Lynch MA (2015) T cells-protective or pathogenic in Alzheimer’s disease? J NeuroImmune Pharmacol 10(4):547–560
McManus RM, Heneka MT (2017) Role of neuroinflammation in neurodegeneration: new insights. Alzheimers Res Ther 9(1):14
Mietelska-Porowska A, Wojda U (2017) T lymphocytes and inflammatory mediators in the interplay between brain and blood in Alzheimer’s disease: potential pools of new biomarkers. J Immunol Res 2017:4626540
International Genomics of Alzheimer’s Disease, C (2015) Convergent genetic and expression data implicate immunity in Alzheimer’s disease. Alzheimers Dement 11(6):658–671
Ramanan VK, Risacher SL, Nho K, Kim S, Shen L, McDonald BC, Yoder KK, Hutchins GD et al (2015) GWAS of longitudinal amyloid accumulation on 18F-florbetapir PET in Alzheimer’s disease implicates microglial activation gene IL1RAP. Brain 138(Pt 10):3076–3088
Askmyr M, Agerstam H, Hansen N, Gordon S, Arvanitakis A, Rissler M, Juliusson G, Richter J et al (2013) Selective killing of candidate AML stem cells by antibody targeting of IL1RAP. Blood 121(18):3709–3713
Driver JA, Zhou XZ, Lu KP (2015) Pin1 dysregulation helps to explain the inverse association between cancer and Alzheimer’s disease. Biochim Biophys Acta 1850(10):2069–2076
Driver JA, Zhou XZ, Lu KP (2014) Regulation of protein conformation by Pin1 offers novel disease mechanisms and therapeutic approaches in Alzheimer’s disease. Discov Med 17(92):93–99
Yarchoan M, James BD, Shah RC, Arvanitakis Z, Wilson RS, Schneider J, Bennett DA, Arnold SE (2017) Association of cancer history with Alzheimer’s disease dementia and neuropathology. J Alzheimers Dis 56(2):699–706
Lu KP, Kondo A, Albayram O, Herbert MK, Liu H, Zhou XZ (2016) Potential of the antibody against cis-phosphorylated tau in the early diagnosis, treatment, and prevention of Alzheimer disease and brain injury. JAMA Neurol 73(11):1356–1362
Lambert JC et al (2013) Meta-analysis of 74,046 individuals identifies 11 new susceptibility loci for Alzheimer’s disease. Nat Genet 45(12):1452–1458
Naj AC, Jun G, Beecham GW, Wang LS, Vardarajan BN, Buros J, Gallins PJ, Buxbaum JD et al (2011) Common variants at MS4A4/MS4A6E, CD2AP, CD33 and EPHA1 are associated with late-onset Alzheimer’s disease. Nat Genet 43(5):436–441
Lamba JK, Pounds S, Cao X, Downing JR, Campana D, Ribeiro RC, Pui CH, Rubnitz JE (2009) Coding polymorphisms in CD33 and response to gemtuzumab ozogamicin in pediatric patients with AML: a pilot study. Leukemia 23(2):402–404
Mortland L, Alonzo TA, Walter RB, Gerbing RB, Mitra AK, Pollard JA, Loken MR, Hirsch B et al (2013) Clinical significance of CD33 nonsynonymous single-nucleotide polymorphisms in pediatric patients with acute myeloid leukemia treated with gemtuzumab-ozogamicin-containing chemotherapy. Clin Cancer Res 19(6):1620–1627
Malik M, Chiles J, Xi HS, Medway C, Simpson J, Potluri S, Howard D, Liang Y et al (2015) Genetics of CD33 in Alzheimer’s disease and acute myeloid leukemia. Hum Mol Genet 24(12):3557–3570
Peng W, Liu C, Xu C, Lou Y, Chen J, Yang Y, Yagita H, Overwijk WW et al (2012) PD-1 blockade enhances T-cell migration to tumors by elevating IFN-gamma inducible chemokines. Cancer Res 72(20):5209–5218
Baruch K, Deczkowska A, Rosenzweig N, Tsitsou-Kampeli A, Sharif AM, Matcovitch-Natan O, Kertser A, David E et al (2016) PD-1 immune checkpoint blockade reduces pathology and improves memory in mouse models of Alzheimer’s disease. Nat Med 22(2):135–137
Sauer CM, Myran DT, Costentin CE, Zwisler G, Safder T, Papatheodorou S, Mucci LA (2018) Effect of long term aspirin use on the incidence of prostate cancer: a systematic review and meta-analysis. Crit Rev Oncol Hematol 132:66–75
Wang J, Tan L, Wang HF, Tan CC, Meng XF, Wang C, Tang SW, Yu JT (2015) Anti-inflammatory drugs and risk of Alzheimer’s disease: an updated systematic review and meta-analysis. J Alzheimers Dis 44(2):385–396
Liby KT, Sporn MB (2016) Rexinoids for prevention and treatment of cancer: opportunities and challenges. Curr Top Med Chem
Cramer PE, Cirrito JR, Wesson DW, Lee CYD, Karlo JC, Zinn AE, Casali BT, Restivo JL et al (2012) ApoE-directed therapeutics rapidly clear beta-amyloid and reverse deficits in AD mouse models. Science 335(6075):1503–1506
Cummings JL, Zhong K, Kinney JW, Heaney C, Moll-Tudla J, Joshi A, Pontecorvo M, Devous M et al (2016) Double-blind, placebo-controlled, proof-of-concept trial of bexarotene Xin moderate Alzheimer’s disease. Alzheimers Res Ther 8:4
Funding
This work was supported by funding from the National Institutes of Health (R01 AG042437, AG051086, AG029672, AG019771, CA129769, R35 CA197289, P30 AG010133, P30 CA082709, and U01 AG24904).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Nudelman, K.N.H., McDonald, B.C., Lahiri, D.K. et al. Biological Hallmarks of Cancer in Alzheimer’s Disease. Mol Neurobiol 56, 7173–7187 (2019). https://doi.org/10.1007/s12035-019-1591-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-019-1591-5