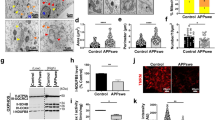
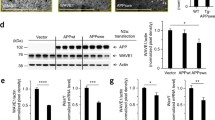
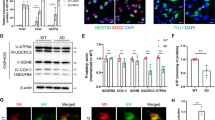
Abstract
A hallmark of Alzheimer’s disease (AD) pathogenesis is the accumulation of extracellular plaques mainly composed of amyloid-β (Aβ) derived from amyloid precursor protein (APP) cleavage. Recent reports suggest that transport of APP in vesicles with huntingtin-associated protein-1 (HAP1) negatively regulates Aβ production. In neurons, HAP1 forms a stable complex with Abelson helper integration site-1 (AHI1), in which mutations cause neurodevelopmental and psychiatric disorders. HAP1 and AHI1 interact with tropomyosin receptor kinases (Trks), which are also associated with APP and mediate neurotrophic signaling. In this study, we hypothesize that AHI1 participates in APP trafficking and processing to rescue AD pathology. Indeed, AHI1 was significantly reduced in mouse neuroblastoma N2a cells expressing human Swedish and Indiana APP (designed as AD model cells) and in 3xTg-AD mouse brain. The AD model cells as well as Ahi1-knockdown cells expressing wild-type APP-695 exhibited a significant reduction in viability. In addition, the AD model cells were reduced in neurite outgrowth. APP C-terminal fragment-β (CTFβ) and Aβ42 were increased in the AD cell lysates and the culture media, respectively. To investigate the mechanism how AHI1 alters APP activities, we overexpressed human AHI1 in the AD model cells. The results showed that AHI1 interacted with APP physically in mouse brain and transfected N2a cells despite APP genotypes. AHI1 expression facilitated intracellular translocation of APP and inhibited APP amyloidogenic process to reduce the level of APP-CTFβ in the total lysates of AD model cells as well as Aβ in the culture media. Consequently, AHI1–APP interactions enhanced neurotrophic signaling through Erk activation and led to restored cell survival and differentiation.






Similar content being viewed by others
Abbreviations
- Aβ:
-
Amyloid-β
- AD:
-
Alzheimer’s disease
- AHI1:
-
Abelson helper integration site-1
- APP:
-
Amyloid precursor protein
- APP-Swe/Ind:
-
Swedish and Indiana APP
- CTF:
-
C-Terminal fragment
- ELISA:
-
Enzyme-linked immunosorbent assay
- Erk:
-
Extracellular signal-regulated protein kinase
- GFP:
-
Green fluorescent protein
- HAP1:
-
Huntingtin-associated protein-1
- PSEN:
-
Presenilin
- sAPPα:
-
Soluble APPα
- Trk:
-
Tropomyosin receptor kinase
References
Brookmeyer R, Johnson E, Ziegler-Graham K, Arrighi HM (2007) Forecasting the global burden of Alzheimer’s disease. Alzheimers Dement 3(3):186–191. https://doi.org/10.1016/j.jalz.2007.04.381
Aisen P, Touchon J, Amariglio R, Andrieu S, Bateman R, Breitner J, Donohue M, Dunn B et al (2017) EU/US/CTAD task force: lessons learned from recent and current Alzheimer’s prevention trials. J Prev Alzheimers Dis 4(2):116–124. https://doi.org/10.14283/jpad.2017.13
Lin CY, Sheu JJ, Tsai IS, Wang ST, Yang LY, Hsu IU, Chang HW, Lee HM et al (2018) Elevated IgM against nepsilon-(carboxyethyl)lysine-modified apolipoprotein A1 peptide 141-147 in Taiwanese with Alzheimer’s disease. Clin Biochem 56:75–82. https://doi.org/10.1016/j.clinbiochem.2018.04.009
Wolfe MS (2003) The secretases of Alzheimer’s disease. Curr Top Dev Biol 54:233–261
Alonso Vilatela ME, Lopez-Lopez M, Yescas-Gomez P (2012) Genetics of Alzheimer’s disease. Arch Med Res 43(8):622–631. https://doi.org/10.1016/j.arcmed.2012.10.017
Citron M, Oltersdorf T, Haass C, McConlogue L, Hung AY, Seubert P, Vigo-Pelfrey C, Lieberburg I et al (1992) Mutation of the beta-amyloid precursor protein in familial Alzheimer’s disease increases beta-protein production. Nature 360(6405):672–674. https://doi.org/10.1038/360672a0
Sosa LJ, Caceres A, Dupraz S, Oksdath M, Quiroga S, Lorenzo A (2017) The physiological role of the amyloid precursor protein as an adhesion molecule in the developing nervous system. J Neurochem 143(1):11–29. https://doi.org/10.1111/jnc.14122
Nalivaeva NN, Turner AJ (2013) The amyloid precursor protein: a biochemical enigma in brain development, function and disease. FEBS Lett 587(13):2046–2054. https://doi.org/10.1016/j.febslet.2013.05.010
Tcw J, Goate AM (2017) Genetics of beta-amyloid precursor protein in Alzheimer’s disease. Cold Spring Harb Perspect Med 7(6). https://doi.org/10.1101/cshperspect.a024539
Zeldenrust SR, Murrell J, Farlow M, Ghetti B, Roses AD, Benson MD (1993) RFLP analysis for APP 717 mutations associated with Alzheimer’s disease. J Med Genet 30(6):476–478
Sinha S, Lieberburg I (1999) Cellular mechanisms of beta-amyloid production and secretion. Proc Natl Acad Sci U S A 96(20):11049–11053
Cai XD, Golde TE, Younkin SG (1993) Release of excess amyloid beta protein from a mutant amyloid beta protein precursor. Science 259(5094):514–516
Tsai YF, Yang DJ, Ngo TH, Shih CH, Wu YF, Lee CK, Phraekanjanavichid V, Yen SF et al (2019) Ganglioside Hp-s1 analogue inhibits amyloidogenic toxicity in Alzheimer’s disease model cells. ACS Chem Neurosci 10:528–536. https://doi.org/10.1021/acschemneuro.8b00406
Wang X, Huang T, Bu G, Xu H (2014) Dysregulation of protein trafficking in neurodegeneration. Mol Neurodegener 9:31. https://doi.org/10.1186/1750-1326-9-31
Salinas S, Bilsland LG, Schiavo G (2008) Molecular landmarks along the axonal route: axonal transport in health and disease. Curr Opin Cell Biol 20(4):445–453. https://doi.org/10.1016/j.ceb.2008.04.002
Stokin GB, Lillo C, Falzone TL, Brusch RG, Rockenstein E, Mount SL, Raman R, Davies P et al (2005) Axonopathy and transport deficits early in the pathogenesis of Alzheimer’s disease. Science 307(5713):1282–1288. https://doi.org/10.1126/science.1105681
Lazarov O, Morfini GA, Pigino G, Gadadhar A, Chen X, Robinson J, Ho H, Brady ST et al (2007) Impairments in fast axonal transport and motor neuron deficits in transgenic mice expressing familial Alzheimer’s disease-linked mutant presenilin 1. J Neurosci 27(26):7011–7020. https://doi.org/10.1523/JNEUROSCI.4272-06.2007
Zhang YW, Chen Y, Liu Y, Zhao Y, Liao FF, Xu H (2013) APP regulates NGF receptor trafficking and NGF-mediated neuronal differentiation and survival. PLoS One 8(11):e80571. https://doi.org/10.1371/journal.pone.0080571
Galvao F Jr, Grokoski KC, da Silva BB, Lamers ML, Siqueira IR (2019) The amyloid precursor protein (APP) processing as a biological link between Alzheimer’s disease and cancer. Ageing Res Rev 49:83–91. https://doi.org/10.1016/j.arr.2018.11.007
Yang GZ, Yang M, Lim Y, Lu JJ, Wang TH, Qi JG, Zhong JH, Zhou XF (2012) Huntingtin associated protein 1 regulates trafficking of the amyloid precursor protein and modulates amyloid beta levels in neurons. J Neurochem 122(5):1010–1022. https://doi.org/10.1111/j.1471-4159.2012.07845.x
Rong J, McGuire JR, Fang ZH, Sheng G, Shin JY, Li SH, Li XJ (2006) Regulation of intracellular trafficking of huntingtin-associated protein-1 is critical for TrkA protein levels and neurite outgrowth. J Neurosci 26(22):6019–6030. https://doi.org/10.1523/JNEUROSCI.1251-06.2006
Lim Y, Wu LL, Chen S, Sun Y, Vijayaraj SL, Yang M, Bobrovskaya L, Keating D et al (2018) HAP1 is required for endocytosis and signalling of BDNF and its receptors in neurons. Mol Neurobiol 55(3):1815–1830. https://doi.org/10.1007/s12035-016-0379-0
Li XJ, Li SH, Sharp AH, Nucifora FC Jr, Schilling G, Lanahan A, Worley P, Snyder SH et al (1995) A huntingtin-associated protein enriched in brain with implications for pathology. Nature 378(6555):398–402. https://doi.org/10.1038/378398a0
Xiang J, Yang H, Zhao T, Sun M, Xu X, Zhou XF, Li SH, Li XJ (2014) Huntingtin-associated protein 1 regulates postnatal neurogenesis and neurotrophin receptor sorting. J Clin Invest 124(1):85–98. https://doi.org/10.1172/JCI69206
Xiang J, Yan S, Li SH, Li XJ (2015) Postnatal loss of hap1 reduces hippocampal neurogenesis and causes adult depressive-like behavior in mice. PLoS Genet 11(4):e1005175. https://doi.org/10.1371/journal.pgen.1005175
Li SH, Yu ZX, Li CL, Nguyen HP, Zhou YX, Deng C, Li XJ (2003) Lack of huntingtin-associated protein-1 causes neuronal death resembling hypothalamic degeneration in Huntington’s disease. J Neurosci 23(17):6956–6964
Ferland RJ, Eyaid W, Collura RV, Tully LD, Hill RS, Al-Nouri D, Al-Rumayyan A, Topcu M et al (2004) Abnormal cerebellar development and axonal decussation due to mutations in AHI1 in Joubert syndrome. Nat Genet 36(9):1008–1013. https://doi.org/10.1038/ng1419
Lotan A, Lifschytz T, Mernick B, Lory O, Levi E, Ben-Shimol E, Goelman G, Lerer B (2017) Alterations in the expression of a neurodevelopmental gene exert long-lasting effects on cognitive-emotional phenotypes and functional brain networks: translational evidence from the stress-resilient Ahi1 knockout mouse. Mol Psychiatry 22(6):884–899. https://doi.org/10.1038/mp.2016.29
Sheng G, Xu X, Lin YF, Wang CE, Rong J, Cheng D, Peng J, Jiang X et al (2008) Huntingtin-associated protein 1 interacts with Ahi1 to regulate cerebellar and brainstem development in mice. J Clin Invest 118(8):2785–2795. https://doi.org/10.1172/JCI35339
Weng L, Lin YF, Li AL, Wang CE, Yan S, Sun M, Gaertig MA, Mitha N et al (2013) Loss of Ahi1 affects early development by impairing BM88/Cend1-mediated neuronal differentiation. J Neurosci 33(19):8172–8184. https://doi.org/10.1523/JNEUROSCI.0119-13.2013
Xu X, Yang H, Lin YF, Li X, Cape A, Ressler KJ, Li S, Li XJ (2010) Neuronal Abelson helper integration site-1 (Ahi1) deficiency in mice alters TrkB signaling with a depressive phenotype. Proc Natl Acad Sci U S A 107(44):19126–19131. https://doi.org/10.1073/pnas.1013032107
Young-Pearse TL, Bai J, Chang R, Zheng JB, LoTurco JJ, Selkoe DJ (2007) A critical function for beta-amyloid precursor protein in neuronal migration revealed by in utero RNA interference. J Neurosci 27(52):14459–14469. https://doi.org/10.1523/JNEUROSCI.4701-07.2007
Canu N, Pagano I, La Rosa LR, Pellegrino M, Ciotti MT, Mercanti D, Moretti F, Sposato V et al (2017) Association of TrkA and APP is promoted by NGF and reduced by cell death-promoting agents. Front Mol Neurosci 10:15. https://doi.org/10.3389/fnmol.2017.00015
Rosenberg PB, Nowrangi MA, Lyketsos CG (2015) Neuropsychiatric symptoms in Alzheimer’s disease: what might be associated brain circuits? Mol Asp Med 43-44:25–37. https://doi.org/10.1016/j.mam.2015.05.005
Amann-Zalcenstein D, Avidan N, Kanyas K, Ebstein RP, Kohn Y, Hamdan A, Ben-Asher E, Karni O et al (2006) AHI1, a pivotal neurodevelopmental gene, and C6orf217 are associated with susceptibility to schizophrenia. Eur J Hum Genet 14(10):1111–1119. https://doi.org/10.1038/sj.ejhg.5201675
Ren L, Qian X, Zhai L, Sun M, Miao Z, Li J, Xu X (2014) Loss of Ahi1 impairs neurotransmitter release and causes depressive behaviors in mice. PLoS One 9(4):e93640. https://doi.org/10.1371/journal.pone.0093640
Xu Z, Guo X, Yang Y, Tucker D, Lu Y, Xin N, Zhang G, Yang L et al (2017) Low-level laser irradiation improves depression-like behaviors in mice. Mol Neurobiol 54(6):4551–4559. https://doi.org/10.1007/s12035-016-9983-2
Wu LL, Zhou XF (2009) Huntingtin associated protein 1 and its functions. Cell Adhes Migr 3(1):71–76
Huang PT, Chen CH, Hsu IU, Salim SA, Kao SH, Cheng CW, Lai CH, Lee CF et al (2015) Huntingtin-associated protein 1 interacts with breakpoint cluster region protein to regulate neuronal differentiation. PLoS One 10(2):e0116372. https://doi.org/10.1371/journal.pone.0116372
Sun J, Roy S (2018) The physical approximation of APP and BACE-1: a key event in Alzheimer’s disease pathogenesis. Dev Neurobiol 78(3):340–347. https://doi.org/10.1002/dneu.22556
Woodruff G, Reyna SM, Dunlap M, Van Der Kant R, Callender JA, Young JE, Roberts EA, Goldstein LS (2016) Defective transcytosis of APP and lipoproteins in human iPSC-derived neurons with familial Alzheimer’s disease mutations. Cell Rep 17(3):759–773. https://doi.org/10.1016/j.celrep.2016.09.034
Liu X, Rothe K, Yen R, Fruhstorfer C, Maetzig T, Chen M, Forrest DL, Humphries RK et al (2017) A novel AHI-1-BCR-ABL-DNM2 complex regulates leukemic properties of primitive CML cells through enhanced cellular endocytosis and ROS-mediated autophagy. Leukemia, UK 31(11):2376–2387. https://doi.org/10.1038/leu.2017.108
Acknowledgements
The authors thank Dr. Yi-Chao Lee and Mr. Jonathan Chang-Cheng Shieh in Taipei Medical University for the helps on N2a cell culture and English editing of the manuscript, respectively.
Funding
This work was supported by the Grants of Taipei Medical University Hospital (101TMU-TMUH-20 to LLT and YFL), the Ministry of Science and Technology in Taiwan (MOST105-2320-B-038-049 to YFL), and National Natural Science Foundation of China and Israel Science Foundation (NSFC-ISF) Joint Research Programme (81461148020 to XJL).
Author information
Authors and Affiliations
Contributions
All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be published. Dr. YFL had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study conception and design: LLT, YFL.
Providing critical materials: LLT, HTL, SL, XJL, YMK.
Acquisition of the data: SFY, THN, IST, FYT, YHT, FYC, YFL.
Analysis and interpretation of the data: HTL, SFY, IST, CKL, SHK, YFL.
Manuscript writing: LLT, HTL, SFY, YFL.
Corresponding author
Ethics declarations
All animal procedures were approved by the Institutional Animal Care and Use Committee in Taipei Medical University (LAC-2015-0191 and LAC-2017-0379).
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
ESM 1
(PDF 227 kb)
Rights and permissions
About this article
Cite this article
Ting, LL., Lu, HT., Yen, SF. et al. Expression of AHI1 Rescues Amyloidogenic Pathology in Alzheimer’s Disease Model Cells. Mol Neurobiol 56, 7572–7582 (2019). https://doi.org/10.1007/s12035-019-1587-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-019-1587-1