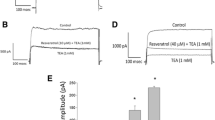
Abstract
Calcium ion dyshomeostasis contributes to the progression of many neurodegenerative diseases and represents a target for the development of neuroprotective therapies, as reported by Duncan et al. (Molecules 15(3):1168–95, 2010), LaFerla (Nat Rev Neurosci 3(11):862–72, 2002), and Niittykoshi et al. (Invest Ophthalmol Vis Sci 51(12):6387–93, 2010). Dysfunctional ryanodine receptors contribute to calcium ion dyshomeostasis and potentially to the pathogenesis of neurodegenerative diseases by generating abnormal calcium ion release from the endoplasmic reticulum, according to Bruno et al. (Neurobiol Aging 33(5):1001 e1–6, 2012) and Stutzmann et al. (J Neurosci 24(2):508–13, 2004). Since ryanodine receptors share functional and structural similarities with potassium channels, as reported by Lanner et al. (Cold Spring Harb Perspect Biol 2(11):a003996, 2010), and small molecules with anti-oxidant properties, such as resveratrol (3,5,4′-trihydroxy-trans-stilbene), directly control the activity of potassium channels, according to Wang et al. (J Biomed Sci 23(1):47, 2016), McCalley et al. (Molecules 19(6):7327–40, 2014), Novakovic et al. (Mol Hum Reprod 21(6):545–51, 2015), Li et al. (Cardiovasc Res 45(4):1035–45, 2000), Gopalakrishnan et al. (Br J Pharmacol 129(7):1323–32, 2000), and Hambrock et al. (J Biol Chem 282(5):3347–56, 2007), we hypothesized that trans-resveratrol can modulate intracellular calcium signaling through direct binding and functional regulation of ryanodine receptors. The goal of our study was to identify and measure the control of ryanodine receptor activity by trans-resveratrol. Mechanisms of calcium signaling mediated by the direct interaction between trans-resveratrol and ryanodine receptors were identified and measured with single-channel electrophysiology. Addition of trans-resveratrol to the cytoplasmic face of the ryanodine receptor increased single-channel activity at physiological and elevated pathophysiological cytoplasmic calcium ion concentrations. The open probability of the channel increases after interacting with the small molecule in a dose-dependent manner, but remains also dependent on the concentration of its physiological ligand, cytoplasmic-free calcium ions. This study provides the first evidence of a direct functional interaction between trans-resveratrol and ryanodine receptors. Such functional control of ryanodine receptors by trans-resveratrol as a novel mechanism of action could provide additional rationales for the development of novel therapeutic strategies to treat and prevent neurodegenerative diseases.







Similar content being viewed by others
References
Brini M, Cali T, Ottolini D, Carafoli E (2014) Neuronal calcium signaling: function and dysfunction. Cell Mol Life Sci 71(15):2787–2814
Berridge MJ (1998) Neuronal calcium signaling. Neuron 21(1):13–26
Contreras L, Drago I, Zampese E, Pozzan T (2010) Mitochondria: the calcium connection. Biochim Biophys Acta 1797(6–7):607–618
Rizzuto R, De Stefani D, Raffaello A, Mammucari C (2012) Mitochondria as sensors and regulators of calcium signalling. Nat. Rev. Mol. Cell Biol. 13(9):566–578
Berridge MJ, Bootman MD, Roderick HL (2003) Calcium signalling: dynamics, homeostasis and remodelling. Nat Rev Mol Cell Biol 4(7):517–529
Clapham DE (2007) Calcium signaling. Cell 131(6):1047–1058
Altschafl BA, Beutner G, Sharma VK, Sheu SS, Valdivia HH (2007) The mitochondrial ryanodine receptor in rat heart: a pharmaco-kinetic profile. Biochim Biophys Acta 1768(7):1784–1795
Beutner D, Voets T, Neher E, Moser T (2001) Calcium dependence of exocytosis and endocytosis at the cochlear inner hair cell afferent synapse. Neuron 29(3):681–690
Blaustein MP, Juhaszova M, Golovina VA, Church PJ, Stanley EF (2002) Na/Ca exchanger and Pmca localization in neurons and astrocytes: functional implications. Ann N Y Acad Sci 976:356–366
De Stefani D, Patron M, Rizzuto R (2015) Structure and function of the mitochondrial calcium uniporter complex. Biochim Biophys Acta 1853(9):2006–2011
Hashambhoy YL, Greenstein JL, Winslow RL (2010) Role of CaMKII in RyR leak, EC coupling and action potential duration: a computational model. J Mol Cell Cardiol 49(4):617–624
Chelu MG, Sarma S, Sood S, Wang S, van Oort RJ, Skapura DG, Li N, Santonastasi M et al (2009) Calmodulin kinase II-mediated sarcoplasmic reticulum Ca2+ leak promotes atrial fibrillation in mice. J Clin Invest 119(7):1940–1951
Kook SY, Jeong H, Kang MJ, Park R, Shin HJ, Han SH, Son SM, Song H et al (2014) Crucial role of calbindin-D28k in the pathogenesis of Alzheimer’s disease mouse model. Cell Death Differ 21(10):1575–1587
Baimbridge KG, Celio MR, Rogers JH (1992) Calcium-binding proteins in the nervous system. Trends Neurosci 15(8):303–308
Heizmann CW, Braun K (1992) Changes in Ca(2+)-binding proteins in human neurodegenerative disorders. Trends Neurosci 15(7):259–264
Wojda U, Salinska E, Kuznicki J (2008) Calcium ions in neuronal degeneration. IUBMB Life 60(9):575–590
Stull JT (2001) Ca2+−dependent cell signaling through calmodulin-activated protein phosphatase and protein kinases minireview series. J Biol Chem 276(4):2311–2312
Verkhratsky A, Petersen OH (2002) The endoplasmic reticulum as an integrating signalling organelle: from neuronal signalling to neuronal death. Eur J Pharmacol 447(2–3):141–154
Koulen P, Janowitz T, Johenning FW, Ehrlich BE (2001) Characterization of the calcium-release channel/ryanodine receptor from zebrafish skeletal muscle. J Membr Biol 183(3):155–163
Marambaud P, Dreses-Werringloer U, Vingtdeux V (2009) Calcium signaling in neurodegeneration. Mol Neurodegener 4:20
Mattson MP (2007) Calcium and neurodegeneration. Aging Cell 6(3):337–350
LaFerla FM (2002) Calcium dyshomeostasis and intracellular signalling in Alzheimer’s disease. Nat Rev Neurosci 3(11):862–872
Niittykoski M, Kalesnykas G, Larsson KP, Kaarniranta K, Akerman KE, Uusitalo H (2010) Altered calcium signaling in an experimental model of glaucoma. Invest Ophthalmol Vis Sci 51(12):6387–6393
Bruno AM, Huang JY, Bennett DA, Marr RA, Hastings ML, Stutzmann GE (2012) Altered ryanodine receptor expression in mild cognitive impairment and Alzheimer’s disease. Neurobiol Aging 33(5):1001 e1–1001 e6
Stutzmann GE, Caccamo A, LaFerla FM, Parker I (2004) Dysregulated Ip3 signaling in cortical neurons of knock-in mice expressing an Alzheimer’s-linked mutation in presenilin1 results in exaggerated Ca2+ signals and altered membrane excitability. J Neurosci 24(2):508–513
Duncan RS, Goad DL, Grillo MA, Kaja S, Payne AJ, Koulen P (2010) Control of intracellular calcium signaling as a neuroprotective strategy. Molecules 15(3):1168–1195
Thibault O, Gant JC, Landfield PW (2007) Expansion of the calcium hypothesis of brain aging and Alzheimer’s disease: minding the store. Aging Cell 6(3):307–317
Gennarelli TA, Graham DI (1998) Neuropathology of the head injuries. Semin Clin Neuropsychiatry 3(3):160–175
Foster TC (2007) Calcium homeostasis and modulation of synaptic plasticity in the aged brain. Aging Cell 6(3):319–325
Brewer LD, Thibault O, Staton J, Thibault V, Rogers JT, Garcia-Ramos G, Kraner S, Landfield PW et al (2007) Increased vulnerability of hippocampal neurons with age in culture: temporal association with increases in Nmda receptor current, Nr2a subunit expression and recruitment of L-type calcium channels. Brain Res 1151:20–31
Buchholz JN, Behringer EJ, Pottorf WJ, Pearce WJ, Vanterpool CK (2007) Age-dependent changes in Ca2+ homeostasis in peripheral neurones: implications for changes in function. Aging Cell 6(3):285–296
Huang T, Gao D, Jiang X, Hu S, Zhang L, Fei Z (2014) Resveratrol inhibits oxygen-glucose deprivation-induced MMP-3 expression and cell apoptosis in primary cortical cells via the Nf-Kappab pathway. Mol Med Rep 10(2):1065–1071
Hogg SJ, Chitcholtan K, Hassan W, Sykes PH, Garrill A (2015) Resveratrol, acetyl-resveratrol, and polydatin exhibit antigrowth activity against 3d cell aggregates of the Skov-3 and Ovcar-8 ovarian cancer cell lines. Obstet Gynecol Int 2015:279591
Murchison D, Griffith WH (2007) Calcium buffering systems and calcium signaling in aged rat basal forebrain neurons. Aging Cell 6(3):297–305
Toescu EC, Verkhratsky A (2007) The importance of being subtle: small changes in calcium homeostasis control cognitive decline in normal aging. Aging Cell 6(3):267–273
Alexianu ME, Ho BK, Mohamed AH, La Bella V, Smith RG, Appel SH (1994) The role of calcium-binding proteins in selective motoneuron vulnerability in amyotrophic lateral sclerosis. Ann Neurol 36(6):846–858
Iacopino AM, Christakos S (1990) Corticosterone regulates calbindin-D28k Mrna and protein levels in rat hippocampus. J Biol Chem 265(18):10177–10180
Lanner JT, Georgiou DK, Joshi AD, Hamilton SL (2010) Ryanodine receptors: structure, expression, molecular details, and function in calcium release. Cold Spring Harb Perspect Biol 2(11):a003996
Hakamata Y, Nakai J, Takeshima H, Imoto K (1992) Primary structure and distribution of a novel ryanodine receptor/calcium release channel from rabbit brain. FEBS Lett 312(2–3):229–235
Rybalchenko V, Grillo MA, Gastinger MJ, Rybalchenko N, Payne AJ, Koulen P (2009) The unliganded long isoform of estrogen receptor beta stimulates brain ryanodine receptor single channel activity alongside with cytosolic Ca2+. J Recept Signal Transduct Res 29(6):326–341
Koulen P, Thrower EC (2001) Pharmacological modulation of intracellular ca(2+) channels at the single-channel level. Mol Neurobiol 24(1–3):65–86
Zhang L, Kelley J, Schmeisser G, Kobayashi YM, Jones LR (1997) Complex formation between junctin, triadin, calsequestrin, and the ryanodine receptor. Proteins of the cardiac junctional sarcoplasmic reticulum membrane. J Biol Chem 272(37):23389–23397
Fill M, Copello JA (2002) Ryanodine receptor calcium release channels. Physiol Rev 82(4):893–922
Copello JA, Barg S, Sonnleitner A, Porta M, Diaz-Sylvester P, Fill M, Schindler H, Fleischer S (2002) Differential activation by Ca2+, Atp and caffeine of cardiac and skeletal muscle ryanodine receptors after block by Mg2+. J Membr Biol 187(1):51–64
Sonnleitner A, Fleischer S, Schindler H (1997) Gating of the skeletal calcium release channel by Atp is inhibited by protein phosphatase 1 but not by Mg2+. Cell Calcium 21(4):283–290
Xu L, Mann G, Meissner G (1996) Regulation of cardiac Ca2+ release channel (ryanodine receptor) by Ca2+, H+, Mg2+, and adenine nucleotides under normal and simulated ischemic conditions. Circ Res 79(6):1100–1109
Bastianetto S, Menard C, Quirion R (2015) Neuroprotective action of resveratrol. Biochim Biophys Acta 1852(6):1195–1201
Marambaud P, Zhao H, Davies P (2005) Resveratrol promotes clearance of Alzheimer’s disease amyloid-beta peptides. J Biol Chem 280(45):37377–37382
Huang TC, Lu KT, Wo YY, Wu YJ, Yang YL (2011) Resveratrol protects rats from Abeta-induced neurotoxicity by the reduction of iNOS expression and lipid peroxidation. PLoS One 6(12):e29102
Rege SD, Geetha T, Griffin GD, Broderick TL, Babu JR (2014) Neuroprotective effects of resveratrol in Alzheimer disease pathology. Front Aging Neurosci 6:218
Renaud S, de Lorgeril M (1992) Wine, alcohol, platelets, and the French paradox for coronary heart disease. Lancet 339(8808):1523–1526
Wu JM, Hsieh TC, Wang Z (2011) Cardioprotection by resveratrol: a review of effects/targets in cultured cells and animal tissues. Am J Cardiovasc Dis 1(1):38–47
Liu BL, Zhang X, Zhang W, Zhen HN (2007) New enlightenment of French paradox: resveratrol’s potential for cancer chemoprevention and anti-cancer therapy. Cancer Biol Ther 6(12):1833–1836
Bastianetto S, Zheng WH, Quirion R (2000) Neuroprotective abilities of resveratrol and other red wine constituents against nitric oxide-related toxicity in cultured hippocampal neurons. Br J Pharmacol 131(4):711–720
Buryanovskyy L, Fu Y, Boyd M, Ma Y, Hsieh TC, Wu JM, Zhang Z (2004) Crystal structure of quinone reductase 2 in complex with resveratrol. Biochemistry 43(36):11417–11426
Sakata Y, Zhuang H, Kwansa H, Koehler RC, Dore S (2010) Resveratrol protects against experimental stroke: putative neuroprotective role of heme oxygenase 1. Exp Neurol 224(1):325–329
Zhang F, Liu J, Shi JS (2010) Anti-inflammatory activities of resveratrol in the brain: role of resveratrol in microglial activation. Eur J Pharmacol 636(1–3):1–7
Gong QH, Wang Q, Shi JS, Huang XN, Liu Q, Ma H (2007) Inhibition of caspases and intracellular free Ca2+ concentrations are involved in resveratrol protection against apoptosis in rat primary neuron cultures. Acta Pharmacol Sin 28(11):1724–1730
Wu XP, Xiong M, Xu CS, Duan LN, Dong YQ, Luo Y, Niu TH, Lu CR (2015) Resveratrol induces apoptosis of human chronic myelogenous leukemia cells in vitro through P38 and Jnk-regulated H2ax phosphorylation. Acta Pharmacol Sin 36(3):353–361
Liao PC, Ng LT, Lin LT, Richardson CD, Wang GH, Lin CC (2010) Resveratrol arrests cell cycle and induces apoptosis in human hepatocellular carcinoma Huh-7 cells. J Med Food 13(6):1415–1423
Varoni EM, Lo Faro AF, Sharifi-Rad J, Iriti M (2016) Anticancer molecular mechanisms of resveratrol. Front Nutr 3:8
Lee BH, Choi SH, Hwang SH, Kim HJ, Lee JH, Nah SY (2013) Resveratrol inhibits GABAC rho receptor-mediated ion currents expressed in Xenopus oocytes. Korean J Physiol Pharmacol 17(2):175–180
Lee BH, Hwang SH, Choi SH, Shin TJ, Kang J, Lee SM, Nah SY (2011) Resveratrol enhances 5-hydroxytryptamine type 3a receptor-mediated ion currents: the role of arginine 222 residue in pre-transmembrane domain I. Biol Pharm Bull 34(4):523–527
Yu L, Wang S, Kogure Y, Yamamoto S, Noguchi K, Dai Y (2013) Modulation of Trp channels by resveratrol and other stilbenoids. Mol Pain 9:3
Wang YJ, Chan MH, Chen L, Wu SN, Chen HH (2016) Resveratrol attenuates cortical neuron activity: roles of large conductance calcium-activated potassium channels and voltage-gated sodium channels. J Biomed Sci 23(1):47
McCalley AE, Kaja S, Payne AJ, Koulen P (2014) Resveratrol and calcium signaling: molecular mechanisms and clinical relevance. Molecules 19(6):7327–7340
Zhang LP, Yin JX, Liu Z, Zhang Y, Wang QS, Zhao J (2006) Effect of resveratrol on L-type calcium current in rat ventricular myocytes. Acta Pharmacol Sin 27(2):179–183
Jakab M, Lach S, Bacova Z, Langeluddecke C, Strbak V, Schmidt S, Iglseder E, Paulmichl M et al (2008) Resveratrol inhibits electrical activity and insulin release from insulinoma cells by block of voltage-gated Ca+ channels and swelling-dependent Cl- currents. Cell Physiol Biochem 22(5–6):567–578
Novakovic R, Radunovic N, Markovic-Lipkovski J, Cirovic S, Beleslin-Cokic B, Ilic B, Ivkovic B, Heinle H et al (2015) Effects of the polyphenol resveratrol on contractility of human term pregnant myometrium. Mol Hum Reprod 21(6):545–551
Li HF, Chen SA, Wu SN (2000) Evidence for the stimulatory effect of resveratrol on Ca(2+)-activated K+ current in vascular endothelial cells. Cardiovasc Res 45(4):1035–1045
Gopalakrishnan M, Molinari EJ, Shieh CC, Monteggia LM, Roch JM, Whiteaker KL, Scott VE, Sullivan JP et al (2000) Pharmacology of human sulphonylurea receptor Sur1 and inward rectifier K(+) channel Kir6.2 combination expressed in Hek-293 cells. Br. J. Pharmacol 129(7):1323–1332
Hambrock A, de Oliveira Franz CB, Hiller S, Grenz A, Ackermann S, Schulze DU, Drews G, Osswald H (2007) Resveratrol binds to the sulfonylurea receptor (Sur) and induces apoptosis in a Sur subtype-specific manner. J Biol Chem 282(5):3347–3356
Samso M, Wagenknecht T, Allen PD (2005) Internal structure and visualization of transmembrane domains of the Ryr1 calcium release channel by cryo-EM. Nat Struct Mol Biol 12(6):539–544
Welch W, Rheault S, West DJ, Williams AJ (2004) A model of the putative pore region of the cardiac ryanodine receptor channel. Biophys J 87(4):2335–2351
Ramachandran S, Serohijos AW, Xu L, Meissner G, Dokholyan NV (2009) A structural model of the pore-forming region of the skeletal muscle ryanodine receptor (Ryr1). PLoS Comput Biol 5(4):e1000367
Kim YH, Kim YS, Kang SS, Cho GJ, Choi WS (2010) Resveratrol inhibits neuronal apoptosis and elevated Ca2+/calmodulin-dependent protein kinase II activity in diabetic mouse retina. Diabetes 59(7):1825–1835
Li W, Wang YP, Gao L, Zhang PP, Zhou Q, Xu QF, Zhou ZW, Guo K et al (2013) Resveratrol protects rabbit ventricular myocytes against oxidative stress-induced arrhythmogenic activity and Ca2+ overload. Acta Pharmacol Sin 34(9):1164–1173
Dong Q, Wu Z, Li X, Yan J, Zhao L, Yang C, Lu J, Deng J et al (2014) Resveratrol ameliorates cardiac dysfunction induced by pressure overload in rats via structural protection and modulation of ca(2+) cycling proteins. J Transl Med 12:323
Kim GH, Kim JE, Rhie SJ, Yoon S (2015) The role of oxidative stress in neurodegenerative diseases. Exp Neurobiol 24(4):325–340
Efremov RG, Leitner A, Aebersold R, Raunser S (2015) Architecture and conformational switch mechanism of the ryanodine receptor. Nature 517(7532):39–43
Song DW, Lee JG, Youn HS, Eom SH, Kim DH (2011) Ryanodine receptor assembly: a novel systems biology approach to 3D mapping. Prog Biophys Mol Biol 105(3):145–161
Ozawa T (2010) Modulation of ryanodine receptor Ca2+ channels (review). Mol Med Rep 3(2):199–204
Zalk R, Clarke OB, des Georges A, Grassucci RA, Reiken S, Mancia F, Hendrickson WA, Frank J et al (2015) Structure of a mammalian ryanodine receptor. Nature 517(7532):44–49
Goldberg DM, Yan J, Soleas GJ (2003) Absorption of three wine-related polyphenols in three different matrices by healthy subjects. Clin Biochem 36(1):79–87
Walle T, Hsieh F, DeLegge MH, Oatis JE Jr, Walle UK (2004) High absorption but very low bioavailability of oral resveratrol in humans. Drug Metab Dispos 32(12):1377–1382
Almeida L, Vaz-da-Silva M, Falcao A, Soares E, Costa R, Loureiro AI, Fernandes-Lopes C, Rocha JF et al (2009) Pharmacokinetic and safety profile of trans-resveratrol in a rising multiple-dose study in healthy volunteers. Mol Nutr Food Res 53 Suppl 1:S7–S15
Walle T (2011) Bioavailability of resveratrol. Ann N Y Acad Sci 1215:9–15
Vitaglione P, Sforza S, Galaverna G, Ghidini C, Caporaso N, Vescovi PP, Fogliano V, Marchelli R (2005) Bioavailability of trans-resveratrol from red wine in humans. Mol Nutr Food Res 49(5):495–504
Kapetanovic IM, Muzzio M, Huang Z, Thompson TN, McCormick DL (2011) Pharmacokinetics, oral bioavailability, and metabolic profile of resveratrol and its dimethylether analog, pterostilbene, in rats. Cancer Chemother Pharmacol 68(3):593–601
Lin HS, Ho PC (2009) A rapid HPLC method for the quantification of 3,5,4′-trimethoxy-trans-stilbene (TMS) in rat plasma and its application in pharmacokinetic study. J Pharm Biomed Anal 49(2):387–392
Funding
Research reported in this publication was supported in part by grants from the National Eye Institute (EY014227 and EY022774), the Institute on Aging (AG027956), and the National Center for Research Resources and National Institute of General Medical Sciences (RR027093) of the National Institutes of Health (PK). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Additional support by the Felix and Carmen Sabates Missouri Endowed Chair in Vision Research and a Challenge Grant from Research to Prevent Blindness (PK) is gratefully recognized.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All procedures involving animals were approved by the institutional animal care and use committee and performed in accordance with institutional and federal guidelines.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
ESM 1
(DOCX 92 kb)
Rights and permissions
About this article
Cite this article
Kraus, J.G., Koulen, P. Resveratrol Directly Controls the Activity of Neuronal Ryanodine Receptors at the Single-Channel Level. Mol Neurobiol 57, 422–434 (2020). https://doi.org/10.1007/s12035-019-01705-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-019-01705-7