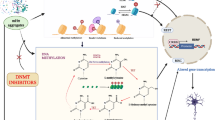
Abstract
Chronic metabolic alterations may represent a risk factor for the development of cognitive impairment, dementia, or neurodegenerative diseases. Hyperglycemia and obesity are known to imprint epigenetic markers that compromise the proper expression of cell survival genes. Here, we showed that chronic hyperglycemia (60 days) induced by a single intraperitoneal injection of streptozotocin compromised cognition by reducing hippocampal ERK signaling and by inducing neurotoxicity in rats. The mechanisms appear to be linked to reduced active DNA demethylation and diminished expression of the neuroprotective transcription factor REST. The impact of the relationship between adiposity and DNA hypermethylation on REST expression was also demonstrated in peripheral blood mononuclear cells in obese children with reduced levels of blood ascorbate. The reversible nature of epigenetic modifications and the cognitive impairment reported in obese children, adolescents, and adults suggest that the correction of the anthropometry and the peripheral metabolic alterations would protect brain homeostasis and reduce the risk of developing neurodegenerative diseases.





Similar content being viewed by others
References
Craft S (2012) Alzheimer disease: insulin resistance and AD extending the translational path. Nat Rev Neurol 8:360–362. https://doi.org/10.1038/nrneurol.2012.112
Crane PK, Walker R, Hubbard RA, Li G, Nathan DM, Zheng H, Haneuse S, Craft S et al (2013) Glucose levels and risk of dementia. N Engl J Med 369:540–548. https://doi.org/10.1056/NEJMoa1215740
Kerti L, Witte AV, Winkler A, Grittner U, Rujescu D, Floel A (2013) Higher glucose levels associated with lower memory and reduced hippocampal microstructure. Neurology 81:1746–1752. https://doi.org/10.1212/01.wnl.0000435561.00234.ee
D’Amelio M, Ragonese P, Callari G et al (2009) Diabetes preceding Parkinson’s disease onset. A case-control study. Park Relat Disord 15:660–664. https://doi.org/10.1016/j.parkreldis.2009.02.013
Boyd AE, Lebovitz HE, Feldman JM (1971) Endocrine function and glucose metabolism in patients with Parkinson’s disease and their alternation by L-Dopa. J Clin Endocrinol Metab 33:829–837. https://doi.org/10.1210/jcem-33-5-829
Brauer R, Bhaskaran K, Chaturvedi N, Dexter DT, Smeeth L, Douglas I (2015) Glitazone treatment and incidence of Parkinson’s disease among people with diabetes: a retrospective cohort study. PLoS Med 12:e1001854. https://doi.org/10.1371/journal.pmed.1001854
Landgrave-Gómez J, Mercado-Gómez O, Guevara-Guzmán R (2015) Epigenetic mechanisms in neurological and neurodegenerative diseases. Front Cell Neurosci 9. https://doi.org/10.3389/fncel.2015.00058
Masliah E, Dumaop W, Galasko D, Desplats P (2013) Identification of concordant epigenetic changes in brain and peripheral blood leukocytes distinctive patterns of DNA methylation associated with Parkinson disease. Epigenetics 8:1030–1038. https://doi.org/10.4161/epi.25865
Smith AR, Smith RG, Condliffe D, Hannon E, Schalkwyk L, Mill J, Lunnon K (2016) Increased DNA methylation near TREM2 is consistently seen in the superior temporal gyrus in Alzheimer’s disease brain. Neurobiol Aging 47:35–40. https://doi.org/10.1016/j.neurobiolaging.2016.07.008
Dallagnol KMC, Remor AP, da Silva RA, Prediger RD, Latini A, Aguiar AS Jr (2016) Running for REST: physical activity attenuates neuroinflammation in the hippocampus of aged mice. Brain Behav Immun 61:31–35. https://doi.org/10.1016/j.bbi.2016.07.159
Lu T, Aron L, Zullo J, Pan Y, Kim H, Chen Y, Yang TH, Kim HM et al (2014) REST and stress resistance in ageing and Alzheimer’s disease. Nature 507:448–454. https://doi.org/10.1038/nature13163
Lenoir da Silva T, Muller Linhares RM, Pertile Remor A, Ghisoni K, de Paula Martins R, Poli A, Aguiar Jr A, Fernando Ronsoni M et al (2017) Blood advanced glycation end products and biomarkers of inflammation in class III obese Brazilian subjects. Integr Obes Diabetes 3:1–4. https://doi.org/10.15761/IOD.1000174
Castro AA, Ghisoni K, Latini A, Quevedo J, Tasca CI, Prediger RDS (2012) Lithium and valproate prevent olfactory discrimination and short-term memory impairments in the intranasal 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) rat model of Parkinson’s disease. Behav Brain Res 229:208–215. https://doi.org/10.1016/j.bbr.2012.01.016
Morris RGM, Garrud P, Rawlins JNP, O’Keefe J (1982) Place navigation impaired in rats with hippocampal lesions. Nature 297:681–683. https://doi.org/10.1038/297681a0
Latini A, Rodriguez M, Borba Rosa R, Scussiato K, Leipnitz G, Reis de Assis D, da Costa Ferreira G, Funchal C et al (2005) 3-Hydroxyglutaric acid moderately impairs energy metabolism in brain of young rats. Neuroscience 135:111–120. https://doi.org/10.1016/j.neuroscience.2005.05.013
Ellman GL (1959) Tissue sulfhydryl groups. Arch Biochem Biophys 82:70–77. https://doi.org/10.1016/0003-9861(59)90090-6
Esterbauer H, Cheeseman KH (1990) Determination of aldehydic lipid peroxidation products: malonaldehyde and 4-hydroxynonenal. Methods Enzymol 186:407–421. https://doi.org/10.1016/0076-6879(90)86134-H
Latremoliere A, Latini A, Andrews N, Cronin SJ, Fujita M, Gorska K, Hovius R, Romero C et al (2015) Reduction of neuropathic and inflammatory pain through inhibition of the tetrahydrobiopterin pathway. Neuron 86:1393–1406. https://doi.org/10.1016/j.neuron.2015.05.033
De Paula Martins R, Glaser V, Da Luz Scheffer D et al (2013) Platelet oxygen consumption as a peripheral blood marker of brain energetics in a mouse model of severe neurotoxicity. J Bioenerg Biomembr 45:449–457. https://doi.org/10.1007/s10863-013-9499-7
Ghisoni K, Martins R de PRDP, Barbeito L, Latini A (2015) Neopterin as a potential cytoprotective brain molecule. J Psychiatr Res 71:134-139. https://doi.org/10.1016/j.jpsychires.2015.10.003
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275. https://doi.org/10.1016/0304-3894(92)87011-4
Trevilatto PC, Line SRP (2000) Use of buccal epithelial cells for PCR amplification of large DNA fragments. J Forensic Odontostomatol 18:6–9
Molognoni F, Cruz AT, Meliso FM, Morais AS, Souza CF, Xander P, Bischof JM, Costa FF et al (2011) Epigenetic reprogramming as a key contributor to melanocyte malignant transformation. Epigenetics 6:450–464. https://doi.org/10.4161/epi.6.4.14917
Xiong Z, Laird PW (1997) COBRA: a sensitive and quantitative DNA methylation assay. Nucleic Acids Res 25:2532–2534. https://doi.org/10.1093/nar/25.12.2532
Sabatino MEME, Petiti JPJP, Del Valle Sosa L et al (2015) Evidence of cellular senescence during the development of estrogen-induced pituitary tumors. Endocr Relat Cancer 22:299–317. https://doi.org/10.1530/ERC-14-0333
Khan A, Khan MI, Iqbal Z, Shah Y, Ahmad L, Nazir S, Watson DG, Khan JA et al (2011) A new HPLC method for the simultaneous determination of ascorbic acid and aminothiols in human plasma and erythrocytes using electrochemical detection. Talanta 84:789–801. https://doi.org/10.1016/j.talanta.2011.02.019
Remor AP, de Matos FJ, Ghisoni K et al (2011) Differential effects of insulin on peripheral diabetes-related changes in mitochondrial bioenergetics: involvement of advanced glycosylated end products. Biochim Biophys Acta. 1812(11):1460–1471. https://doi.org/10.1016/j.bbadis.2011.06.017
Sindelar DK, Havel PJ, Seeley RJ, Wilkinson CW, Woods SC, Schwartz MW (2000) Low plasma leptin levels contribute to diabetic hyperphagia in rats. Diabetes 48:1275–1280. https://doi.org/10.2337/diabetes.48.6.1275
Thornalley PJ, Hooper NI, Jennings PE, Florkowski CM, Jones AF, Lunec J, Barnett AH (1989) The human red blood cell glyoxalase system in diabetes mellitus. Diabetes Res Clin Pract 7:115–120. https://doi.org/10.1016/0168-8227(89)90101-0
Takahashi K (1977) Further studies on the reactions of phenylglyoxal and related reagents with proteins. J Biochem 81:403–414
Boitard C, Cavaroc A, Sauvant J, Aubert A, Castanon N, Layé S, Ferreira G (2014) Impairment of hippocampal-dependent memory induced by juvenile high-fat diet intake is associated with enhanced hippocampal inflammation in rats. Brain Behav Immun 40:9–17. https://doi.org/10.1016/j.bbi.2014.03.005
Zhou X, Zhang J, Chen Y, Ma T, Wang Y, Wang J, Zhang Z (2014) Aggravated cognitive and brain functional impairment in mild cognitive impairment patients with type 2 diabetes: a resting-state functional MRI study. J Alzheimers Dis 41:925–935. https://doi.org/10.3233/JAD-132354
Air EL, Strowski MZ, Benoit SC, Conarello SL, Salituro GM, Guan XM, Liu K, Woods SC et al (2002) Small molecule insulin mimetics reduce food intake and body weight and prevent development of obesity. Nat Med 8:179–183. https://doi.org/10.1038/nm0202-179
Werther GA, Hogg A, Oldfield BJ et al (1987) Localization and characterization of insulin receptors in rat brain and pituitary gland using in vitro autoradiography and computerized densitometry. Endocrinology 121:1562–1570. https://doi.org/10.1210/endo-121-4-1562
Tomlinson DR, Gardiner NJ (2008) Glucose neurotoxicity. Nat Rev Neurosci 9:36–45. https://doi.org/10.1038/nrn2294
Haj-ali V, Mohaddes G, Babri SH (2009) Intracerebroventricular insulin improves spatial learning and memory in male Wistar rats. Behav Neurosci 123:1309–1314. https://doi.org/10.1037/a0017722
Pardo J, Uriarte M, Cónsole GM, Reggiani PC, Outeiro TF, Morel GR, Goya RG (2016) Insulin-like growth factor-I gene therapy increases hippocampal neurogenesis, astrocyte branching and improves spatial memory in female aging rats. Eur J Neurosci 44:2120–2128. https://doi.org/10.1111/ejn.13278
Banks WA, Jaspan JB, Huang W, Kastin AJ (1997) Transport of insulin across the blood-brain barrier: saturability at euglycemic doses of insulin. Peptides 18:1423–1429. https://doi.org/10.1016/S0196-9781(97)00231-3
Craft S (2006) Insulin resistance syndrome and Alzheimer disease: pathophysiologic mechanisms and therapeutic implications. Alzheimer Dis Assoc Disord 20:298–301. https://doi.org/10.1097/01.wad.0000213866.86934.7e
Belfiore A, Frasca F, Pandini G, Sciacca L, Vigneri R (2009) Insulin receptor isoforms and insulin receptor/insulin-like growth factor receptor hybrids in physiology and disease. Endocr Rev 30:586–623
Atkins CM, Selcher JC, Petraitis JJ, Trzaskos JM, Sweatt JD (1998) The MAPK cascade is required for mammalian associative learning. Nat Neurosci 1:602–609. https://doi.org/10.1038/2836
Blum S, Moore AN, Adams F, Dash PK (1999) A mitogen-activated protein kinase cascade in the CA1/CA2 subfield of the dorsal hippocampus is essential for long-term spatial memory. J Neurosci 19:3535–3544. https://doi.org/10.1002/hipo.10070
Lang C, Barco A, Zablow L, Kandel ER, Siegelbaum SA, Zakharenko SS (2004) Transient expansion of synaptically connected dendritic spines upon induction of hippocampal long-term potentiation. Proc Natl Acad Sci 101:16665–16670. https://doi.org/10.1073/pnas.0407581101
Bechara RG, Lyne R, Kelly ÁM (2014) BDNF-stimulated intracellular signalling mechanisms underlie exercise-induced improvement in spatial memory in the male Wistar rat. Behav Brain Res 275:297–306. https://doi.org/10.1016/j.bbr.2013.11.015
Bekinschtein P, Cammarota M, Igaz LM, Bevilaqua LRM, Izquierdo I, Medina JH (2007) Persistence of long-term memory storage requires a late protein synthesis- and BDNF-dependent phase in the hippocampus. Neuron 53:261–277. https://doi.org/10.1016/j.neuron.2006.11.025
Ortíz BM, Emiliano JR, Ramos-Rodríguez E et al (2016) Brain-derived neurotrophic factor plasma levels and premature cognitive impairment/dementia in type 2 diabetes. World J Diabetes 7:615–620. https://doi.org/10.4239/wjd.v7.i20.615
Spetter MS, Hallschmid M (2015) Intranasal neuropeptide administration to target the human brain in health and disease. Mol Pharm 12:2767–2780. https://doi.org/10.1021/acs.molpharmaceut.5b00047
Pham CLL, Cappai R (2013) The interplay between lipids and dopamine on α-synuclein oligomerization and membrane binding. Biosci Rep 33:807–814. https://doi.org/10.1042/BSR20130092
Gilkerson RW, De vries RLA, Lebot P et al (2012) Mitochondrial autophagy in cells with mtDNA mutations results from synergistic loss of transmembrane potential and mTORC1 inhibition. Hum Mol Genet 21:978–990. https://doi.org/10.1093/hmg/ddr529
Renna M, Jimenez-Sanchez M, Sarkar S, Rubinsztein DC (2010) Chemical inducers of autophagy that enhance the clearance of mutant proteins in neurodegenerative diseases. J Biol Chem 285:11061–11067
Schlensog M, Magnus L, Heide T, Eschenbruch J, Steib F, Tator M, Kloten V, Rose M et al (2016) Epigenetic loss of putative tumor suppressor SFRP3 correlates with poor prognosis of lung adenocarcinoma patients. Epigenetics 0:1–14. https://doi.org/10.1080/15592294.2016.1229730
Goodier JL, Kazazian HH (2008) Retrotransposons revisited: the restraint and rehabilitation of parasites. Cell 135:23–35. https://doi.org/10.1016/j.cell.2008.09.022
Bollati V, Galimberti D, Pergoli L, Dalla Valle E, Barretta F, Cortini F, Scarpini E, Bertazzi PA et al (2011) DNA methylation in repetitive elements and Alzheimer disease. Brain Behav Immun 25:1078–1083. https://doi.org/10.1016/j.bbi.2011.01.017
Song Z, Shah SZA, Yang W, Dong H, Yang L, Zhou X, Zhao D (2017) Downregulation of the repressor element 1-silencing transcription factor (REST) is associated with Akt-mTOR and Wnt-β-catenin signaling in prion diseases models. Front Mol Neurosci 10:1–18. https://doi.org/10.3389/fnmol.2017.00128
Song Z, Zhu T, Zhou X, Barrow P, Yang W, Cui Y, Yang L, Zhao D (2016) REST alleviates neurotoxic prion peptide-induced synaptic abnormalities neurofibrillary degeneration and neuronal death partially via LRP6-mediated Wnt-β-catenin signaling. Oncotarget 7:12035–12052. https://doi.org/10.18632/oncotarget.7640
Lu T, Aron L, Zullo J, Pan Y, Kim H, Chen Y, Yang TH, Kim HM et al (2014) REST and stress resistance in ageing and Alzheimer disease. Nature 507:448–454. https://doi.org/10.1038/nature13163
Nho K, Kim S, Risacher SL, Shen L, Corneveaux JJ, Swaminathan S, Lin H, Ramanan VK et al (2015) Protective variant for hippocampal atrophy identified by whole exome sequencing. Ann Neurol 77:547–552. https://doi.org/10.1002/ana.24349
Yu M, Cai L, Liang M, Huang Y, Gao H, Lu S, Fei J, Huang F (2009) Alteration of NRSF expression exacerbating 1-methyl-4-phenyl-pyridinium ion-induced cell death of SH-SY5Y cells. Neurosci Res 65:236–244. https://doi.org/10.1016/j.neures.2009.07.006
Sunahori K, Juang Y-T, Tsokos GC (2009) Methylation status of CpG islands flanking a cAMP response element motif on the protein phosphatase 2Ac alpha promoter determines CREB binding and activity. J Immunol 182:1500–1508
Huang Y, Myers SJ, Dingledine R (1999) Transcriptional repression by REST: recruitment of Sin3A and histone deacetylase to neuronal genes. Nat Neurosci 2:867–872. https://doi.org/10.1038/13165
Boulle F, van den Hove DLA, Jakob SB, Rutten BP, Hamon M, van Os J, Lesch KP, Lanfumey L et al (2012) Epigenetic regulation of the BDNF gene: implications for psychiatric disorders. Mol Psychiatry 17:584–596. https://doi.org/10.1038/mp.2011.107
Okano M, Bell DW, Haber DA, Li E (1999) DNA methyltransferases Dnmt3a and Dnmt3b are essential for de novo methylation and mammalian development. Cell 99:247–257. https://doi.org/10.1016/S0092-8674(00)81656-6
Hermann A, Goyal R, Jeltsch A (2004) The Dnmt1 DNA-(cytosine-C5)-methyltransferase methylates DNA processively with high preference for hemimethylated target sites. J Biol Chem 279:48350–48359. https://doi.org/10.1074/jbc.M403427200
Wu SC, Zhang Y (2010) Active DNA demethylation: many roads lead to Rome. Nat Rev Mol Cell Biol 11:750–750. https://doi.org/10.1038/nrm2975
Minor EA, Court BL, Young JI, Wang G (2013) Ascorbate induces ten-eleven translocation (Tet) methylcytosine dioxygenase-mediated generation of 5-hydroxymethylcytosine. J Biol Chem 288:13669–13674. https://doi.org/10.1074/jbc.C113.464800
Zhao Y, Zhu M, Yu Y, Qiu L, Zhang Y, He L, Zhang J (2016) Brain REST/NRSF is not only a silent repressor but also an active protector. Mol Neurobiol 54:1–10. https://doi.org/10.1007/s12035-015-9658-4
Palm K, Belluardo N, Metsis M, Timmusk T (1998) Neuronal expression of zinc finger transcription factor REST/NRSF/XBR gene. J Neurosci 18:1280–1296
Dick KJ, Nelson CP, Tsaprouni L, Sandling JK, Aïssi D, Wahl S, Meduri E, Morange PE et al (2014) DNA methylation and body-mass index: a genome-wide analysis. Lancet (London, England) 383:1990–1998. https://doi.org/10.1016/S0140-6736(13)62674-4
Tsai CK, Kao TW, Lee JT, Wu CJ, Hueng DY, Liang CS, Wang GC, Yang FC et al (2016) Increased risk of cognitive impairment in patients with components of metabolic syndrome. Med 95:e4791. https://doi.org/10.1097/MD.0000000000004791
Bozkurt H, Özer S, Yılmaz R, Sönmezgöz E, Kazancı Ö, Erbaş O, Demir O (2016) Assessment of neurocognitive functions in children and adolescents with obesity. Appl Neuropsychol Child 2965:1–7. https://doi.org/10.1080/21622965.2016.1150184
El-Osta A, Brasacchio D, Yao D et al (2008) Transient high glucose causes persistent epigenetic changes and altered gene expression during subsequent normoglycemia. J Exp Med 205:2409–2417. https://doi.org/10.1084/jem.20081188
Roth TL, Sweatt JD (2009) Regulation of chromatin structure in memory formation. Curr Opin Neurobiol 19:336–342
Acknowledgments
The authors are grateful to Theodore Griswold for language editing.
Funding
This work was supported by grants from Conselho Nacional de Desenvolvimento Científico e Tecnológico, Brazil (479222/2013-4); Coordenação de Aperfeiçoamento de Pessoal de Nível Superior, Brazil (PVE: 004_2013 and 88881.062164/2014-01, INCT 189405 16/2014); Programa de Apoio a Núcleos de Excelência PRONEX (NENASC Project); Secretaría de Ciencia y Técnica de la Universidad Nacional de Córdoba, Argentina; Fondo para la Investigación Científica y Tecnológica, Argentina; and Consejo Nacional de Investigaciones Científicas y Técnicas, Argentina. R.D.P., A.S.A.Jr., R.W. and A.L. are CNPq fellows.
Author information
Authors and Affiliations
Contributions
A.P.R. designed, planned, and performed the animal model; carried out biochemical measurements, Western blot assays, and data analyses; prepared the initial figures; and revised the manuscript. R.A.S. designed and performed the animal model; carried out molecular assays, epigenetic measurements, and data analyses; and revised the manuscript. F.J.M. set up the animal model and performed the PCR assays; V.G. carried out histochemical and PCR analyses; R.D.P performed Western blot assays and HPLC measurements; D.L.S., D.P., and D.C.A. performed DNA studies and data analyses; A.R. coordinated insulin-related Western blot studies and insulin measurements; P.A.O. performed behavioral tests; M.F.R., A.H., R.M.M.L., and R.W. were responsible for the clinical data from obese and diabetic patients, collected the samples, and were responsible for funding acquisition; R.D.P. coordinated the behavioral tests and revised the manuscript; A.P.S. performed the DNA extraction and revised the manuscript; A.S.A.Jr. performed statistics, revised the manuscript, and was responsible for funding acquisition; A.T. and A.L.D.P. performed the immunoelectron microscopy assays and was responsible for funding acquisition; A.L. coordinated all experimental procedures and data analyses, prepared the final version of the figures, wrote the manuscript, was responsible for funding acquisition, and provided the required infrastructure.
Corresponding author
Ethics declarations
All procedures detailed in this study employing experimental animals were performed under the ethical guidelines of the Ethics Committee for Animal Research (PP00350/CEUA) of the Universidade Federal de Santa Catarina, Florianópolis, SC, Brazil, and were carried out in accordance with the ARRIVE guidelines. All the human experiments were performed under the ethical guidelines of the Ethics Committee for Human Research (CEPSH/435/09, CEPSH/2150/2011, and CAAE 33173314.7.0000.0121) of the Universidade Federal de Santa Catarina, Florianópolis, SC, Brazil, and subjects’ consent was obtained according to the Declaration of Helsinki.
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Highlights
• Impaired cognition and hippocampal ERK signaling were induced in hyperglycemic rats.
• Chronic metabolic disturbances elicited neurotoxicity with signs of neurodegeneration.
• Hyperglycemia elicited reduced hippocampal DNA demethylation with REST inactivation.
• Leukocytes from obese children showed repressed REST and DNA hypermethylation.
Rights and permissions
About this article
Cite this article
Remor, A.P., da Silva, R.A., de Matos, F.J. et al. Chronic Metabolic Derangement-Induced Cognitive Deficits and Neurotoxicity Are Associated with REST Inactivation. Mol Neurobiol 56, 1539–1557 (2019). https://doi.org/10.1007/s12035-018-1175-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-018-1175-9