Abstract
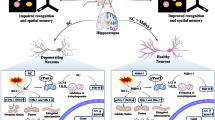
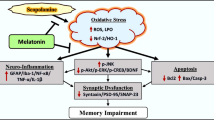
Our previous report on hippocampal proteome analysis suggested the involvement of voltage-dependent anion channel (Vdac) 1 in scopolamine-induced amnesia. Further silencing of Vdac1 in young mice reduced the recognition memory. Vdac1 is a porin protein present abundantly on outer mitochondrial membrane. It acts as a transporter of energy metabolites ATP/ADP and Ca2+ ions and helps in communication between mitochondrial matrix and cytosol. As Vdac1-associated energy metabolism may be affected during amnesia, we determined the downstream function of Vdac1 in the present study. The expression of Vdac1 and total ATP level was decreased in the hippocampus of scopolamine-induced amnesic mice. Also, the mitochondrial membrane potential, cristae organization, and morphology were disrupted leading to increased ROS generation and reduced SOD and catalase activity. On the other hand, there was increase in the expression of pro-apoptotic marker proteins (Bax, Bad, Casp 3), leading to rising degenerated neuronal cells in the dentate gyrus and Cornu ammonis 3 and 1 subregions of the hippocampus during amnesia. Further, to check whether Vdac1 downregulation is associated with neurodegeneration, we infused Vdac1 siRNA stereotaxically in the hippocampus of normal young mice. As compared to control, Vdac1 silencing decreased ATP level and mitochondrial membrane potential leading to increase in the number of degenerated neuronal cells in subregions of the hippocampus. Taken together, our study shows that downregulation of Vdac1 causes neurodegeneration through mitochondrial disintegration in the hippocampus of scopolamine-induced amnesic mice.








Similar content being viewed by others
References
Blokland A (2005) Scopolamine-induced deficits in cognitive performance: a review of animal studies. Scopolamine Rev 1:1–76
Konar A, Shah N, Singh R, Saxena N, Kaul SC, Wadhwa R, Thakur MK (2011) Protective role of ashwagandha leaf extract and its component withanone on scopolamine-induced changes in the brain and brain-derived cells. PLoS One 6(11):e27265
Gautam A, Wadhwa R, Thakur MK (2013) Involvement of hippocampal arc in amnesia and its recovery by alcoholic extract of ashwagandha leaves. Neurobiol Learn Mem 106:177–184
Baghel MS, Thakur MK (2017) Differential proteome profiling in the hippocampus of amnesic mice. Hippocampus 27:845–859
Weeber EJ, Levy M, Sampson MJ, Anflous K, Armstrong DL, Brown SE, Sweatt JD, Craigen WJ (2002) The role of mitochondrial porins and the permeability transition pore in learning and synaptic plasticity. J Biol Chem 277:18891–18897
Huang H, Shah K, Bradbury NA, Li C, White C (2014) Mcl-1 promotes lung cancer cell migration by directly interacting with Vdac to increase mitochondrial Ca2+ uptake and reactive oxygen species generation. Cell Death Dis 5:e1482
Okada SF, O'Neal WK, Huang P, Nicholas RA, Ostrowski LE, Craigen WJ, Lazarowski ER, Boucher RC (2004) Voltage-dependent anion channel-1 (VDAC-1) contributes to ATP release and cell volume regulation in murine cells. J Gen Physiol 124:513–526
Kapogiannis D, Mattson MP (2011) Disrupted energy metabolism and neuronal circuit dysfunction in cognitive impairment and Alzheimer's disease. Lancet Neurol 10:187–198
Pathak D, Shields LY, Mendelsohn BA, Haddad D, Lin W, Gerencser AA, Kim H, Brand MD et al (2015) The role of mitochondrially derived ATP in synaptic vesicle recycling. J Biol Chem 290:22325–22236
Marsicano G, Lafenêtre P (2009) Roles of the endocannabinoid system in learning and memory. Curr Top Behav Neurosci 1:201–230
Broyd SJ, van Hell HH, Beale C, Yücel M, Solowij N (2016) Acute and chronic effects of cannabinoids on human cognition—a systematic review. Biol Psychiatry 79:557–567
Hebert-Chatelain E, Desprez T, Serrat R, Bellocchio L, Soria-Gomez E, Busquets-Garcia A, Pagano Zottola AC, Delamarre A et al (2016) A cannabinoid link between mitochondria and memory. Nature 539:555–559
Hagihara H, Toyama K, Yamasaki N, Miyakawa T (2009) Dissection of hippocampal dentate gyrus from adult mouse. J Vis Exp 17. https://doi.org/10.3791/1543
Ji XF, Chi TY, Xu Q, He XL, Zhou XY, Zhang R, Zou LB (2014) Xanthoceraside ameliorates mitochondrial dysfunction contributing to the improvement of learning and memory impairment in mice with intracerebroventricular injection of aβ1–42. Evid Based Complement Alternat Med 2014:969342
Premkumar A, Simantov R (2002) Mitochondrial voltage-dependent anion channel is involved in dopamine-induced apoptosis. J Neurochem 82:345–352
Prins JM, Park S, Lurie D (2010) Decreased expression of the voltage-dependent anion channel in differentiated PC-12 and SH-SY5Y cells following low-level Pb exposure. Toxicol Sci 113:169–176
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Chida J, Yamane K, Takei T, Kido H (2012) An efficient extraction method for quantitation of adenosine triphosphate in mammalian tissues and cells. Anal Chim Acta 727:8–12
Paramanik V, Thakur MK (2012) Estrogen receptor β and its domains interact with casein kinase 2, phosphokinase C, and N-myristoylation sites of mitochondrial and nuclear proteins in mouse brain. J Biol Chem 287:22305–22316
Jain K, Prasad D, Singh SB, Kohli E (2015) Hypobaric hypoxia imbalances mitochondrial dynamics in rat brain hippocampus. Neurol Res Int 2015:742059
Saetersdal T, Engedal H, Røli J, Myklebust R (1980) Calcium and magnesium levels in isolated mitochondria from human cardiac biopsies. Histochemistry 68:1–8
Mishra VK, Upadhyay AR, Pandey SK, Tripathi BD (2008) Concentrations of heavy metals and aquatic macrophytes of Govind Ballabh Pant Sagar an anthropogenic lake affected by coal mining effluent. Environ Monit Assess 141:49–58
Das L, Vinayak M (2014) Long term effect of curcumin in regulation of glycolytic pathway and angiogenesis via modulation of stress activated genes in prevention of cancer. PLoS One 9:e99583
Weydert CJ, Cullen JJ (2010) Measurement of superoxide dismutase, catalase and glutathione peroxidase in cultured cells and tissue. Nat Protoc 5:51–66
Qiu JH, Asai A, Chi S, Saito N, Hamada H, Kirino T (2000) Proteasome inhibitors induce cytochrome c-caspase-3-like protease-mediated apoptosis in cultured cortical neurons. J Neurosci 20:259–265
Schmued LC, Stowers CC, Scallet AC, Xu L (2005) Fluoro-Jade C results in ultra high resolution and contrast labeling of degenerating neurons. Brain Res 1035:24–31
Ehara A, Ueda S (2009) Application of Fluoro-Jade C in acute and chronic neurodegeneration models: utilities and staining differences. Acta Histochem Cytochem 42:171–179
Klein AM, Ferrante RJ (2007) The neuroprotective role of creatine. Creatine and creatine kinase in health and disease. Springer, Berlin, pp 205–243
Huizing M, DePinto V, Ruitenbeek W, Trijbels FJ, van den Heuvel LP, Wendel U (1996) Importance of mitochondrial transmembrane processes in human mitochondriopathies. J Bioenerg Biomembr 28:109–114
Anflous K, Armstrong DD, Craigen WJ (2001) Altered mitochondrial sensitivity for ADP and maintenance of creatine-stimulated respiration in oxidative striated muscles from VDAC1-deficient mice. J Biol Chem 276:1954–1960
Anflous-Pharayra K, Lee N, Armstrong DL, Craigen WJ (2011) VDAC3 has differing mitochondrial functions in two types of striated muscles. Biochim Biophys Acta 1807:150–156
Loubiere C, Clavel S, Gilleron J, Harisseh R, Fauconnier J, Ben-Sahra I, Kaminski L, Laurent K et al (2017) The energy disruptor metformin targets mitochondrial integrity via modification of calcium flux in cancer cells. Sci Rep 7:5040
Carvalho C, Correia SC, Cardoso S, Plácido AI, Candeias E, Duarte AI, Moreira PI (2015) The role of mitochondrial disturbances in Alzheimer, Parkinson and Huntington diseases. Expert Rev Neurother 15:867–884
Sheridan C, Martin SJ (2010) Mitochondrial fission/fusion dynamics and apoptosis. Mitochondrion 10:640–648
Chinopoulos C, Adam-Vizi V (2010) Mitochondrial Ca2+ sequestration and precipitation revisited. FEBS J 277:3637–3651
Carafoli E (2010) The fateful encounter of mitochondria with calcium: how did it happen? Biochim Biophys Acta 1797:595–606
Pivovarova NB, Nguyen HV, Winters CA, Brantner CA, Smith CL, Andrews SB (2004) Excitotoxic calcium overload in a subpopulation of mitochondria triggers delayed death in hippocampal neurons. J Neurosci 24:5611–5622
Wang CH, Wu SB, Wu YT, Wei YH (2013) Oxidative stress response elicited by mitochondrial dysfunction: implication in the pathophysiology of aging. Exp Biol Med 238:450–460
Guo JY, Xia B, White E (2013) Autophagy-mediated tumor promotion. Cell 155:1216–1219
Maharjan S, Oku M, Tsuda M, Hoseki J, Sakai Y (2014) Mitochondrial impairment triggers cytosolic oxidative stress and cell death following proteasome inhibition. Sci Rep 4:5896
Vander Heiden MG, Chandel NS, Williamson EK, Schumacker PT, Thompson CB (1997) Bcl-xL regulates the membrane potential and volume homeostasis of mitochondria. Cell 91:627–637
Narita M, Shimizu S, Ito T, Chittenden T, Lutz RJ, Matsuda H, Tsujimoto Y (1998) Bax interacts with the permeability transition pore to induce permeability transition and cytochrome c release in isolated mitochondria. Proc Natl Acad Sci U S A 95:14681–14686
Arbel N, Ben-Hail D, Shoshan-Barmatz V (2012) Mediation of the antiapoptotic activity of Bcl-xL protein upon interaction with VDAC1 protein. J Biol Chem 287:23152–23161
Arif T, Vasilkovsky L, Refaely Y, Konson A, Shoshan-Barmatz V (2014) Silencing VDAC1 expression by siRNA inhibits cancer cell proliferation and tumor growth in vivo. Mol Ther Nucleic Acids 3:e159
Dubey AK, Godbole A, Mathew MK (2016) Regulation of VDAC trafficking modulates cell death. Cell Death Discov 2:16085
Acknowledgements
Meghraj Singh Baghel thanks University Grants Commission-Centre for Advanced Study, Department of Zoology, for the award of Senior Research Fellowship. We thank Prof. TC Nag, All India Institute of Medical Sciences, New Delhi, for discussing the TEM results.
Funding
Financial support from the Department of Biotechnology, Department of Science and Technology, and Indian Council of Medical Research, Government of India, are highly acknowledged.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Baghel, M.S., Thakur, M.K. Vdac1 Downregulation Causes Mitochondrial Disintegration Leading to Hippocampal Neurodegeneration in Scopolamine-Induced Amnesic Mice. Mol Neurobiol 56, 1707–1718 (2019). https://doi.org/10.1007/s12035-018-1164-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-018-1164-z