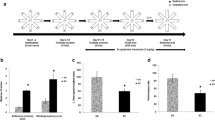
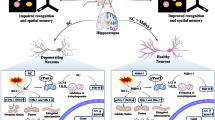
Abstract
Histone post-translational modifications play an important role in the regulation of long-term memory and modulation of expression of neuronal immediate early genes (IEGs). The lysine methyltransferase KMT1A/ Suv39h1 (a mammalian ortholog of the Drosophila melanogaster SU (VAR) 3-9) aids in the methylation of histone H3 at lysine 9. We previously reported that age-related memory decline is associated with an increase in Suv39h1 expression in the hippocampus of male mice. The scopolamine-induced amnesic mouse model is a well-known animal model of memory impairment. In the current study, we have made an attempt to find a link between the changes in the H3K9 trimethylation pattern and memory decline during scopolamine-induced amnesia. It was followed by checking the effect of siRNA-mediated silencing of hippocampal Suv39h1 on memory and expression of neuronal IEGs. Scopolamine treatment significantly increased global levels of H3K9me3 and Suv39h1 in the amnesic hippocampus. Suv39h1 silencing in amnesic mice reduced H3K9me3 levels at the neuronal IEGs (Arc and BDNF) promoter, increased the expression of Arc and BDNF in the hippocampus, and improved recognition memory. Thus, these findings suggest that the silencing of Suv39h1 alone or in combination with other epigenetic drugs might be effective for treating memory decline during amnesia.







Similar content being viewed by others
Data Availability
The data presented in the article are original.
References
Gautam A, Wadhwa R, Thakur MK (2013) Involvement of hippocampal arc in amnesia and its recovery by alcoholic extract of Ashwagandha leaves. Neurobiol Learn Mem 106:177–184. https://doi.org/10.1016/j.nlm.2013.08.009
Konar A, Shah N, Singh R, Saxena N, Kaul SC, Wadhwa R, Thakur MK (2011) Protective role of Ashwagandha leaf extract and its component with anone on scopolamine-induced changes in the brain and brain-derived cells. PloS One 6(11):e27265. https://doi.org/10.1371/journal.pone.0027265
Singh P, Konar A, Kumar A, Srivas S, Thakur MK (2015) Hippocampal chromatin modifying enzymes are pivotal for scopolamine-induced synaptic plasticity gene expression changes and memory impairment. J Neurochem 134:642–651 srivas
Yadang FSA, Nguezeye Y, Kom CW, Betote PHD, Mamat A, Tchokouaha LRY, Taiwé GS, Agbor GA et al (2020) Scopolamine-induced memory impairment in mice: neuroprotective effects of Carissa edulis (Forssk.) Valh (Apocynaceae) aqueous extract. Int. J Alzheimers Dis 2020:6372059 8. https://doi.org/10.1155/2020/6372059
Caffaro PA, Suárez LD, Blake MG, Delorenzi A (2012) Dissociation between memory reactivation and its behavioral expression: scopolamine interferes with memory expression without disrupting long-term storage. Neurobiol Learn Mem 98:235–245. https://doi.org/10.1016/j.nlm.2012.08.003
Klinkenberg I, Blokland A (2010) The validity of scopolamine as a pharmacological model for cognitive impairment: a review of animal behavioral studies. Neurosci Biobehav Rev 34:1307–1350. https://doi.org/10.1016/j.neubiorev.2010.04.001
Brouillette J, Young D, During MJ, Quirion R (2007) Hippocampal gene expression profiling reveals the possible involvement of Homer1 and GABAB receptors in Scopolamine-induced amnesia. J Neurochem 102:1978–1989. https://doi.org/10.1111/j.1471-4159.2007.04666.x
Balmus IM, Ciobica A (2017) Main plant extracts’ active properties effective on scopolamine-induced memory loss. Am J Alzheimers Dis Other Demen 32:418–428. https://doi.org/10.1177/1533317517715906
Hsieh MT, Hsieh CL, Lin LW, Wu CR, Huang GS (2003) Differential gene expression of scopolamine-treated rat hippocampus-application of cDNA microarray technology. Life Sci 73:1007–1016. https://doi.org/10.1016/s0024-3205(03)00372-2
Konar A, Thakur MK (2015) Neuropsin expression correlates with dendritic marker MAP2c level in different brain regions of aging mice. Mol Neurobiol 51:1130–1138. https://doi.org/10.1007/s12035-014-8780-z
Srivas S, Thakur MK (2019) Epigenetic regulation of neuronal immediate early genes is associated with decline in their expression and memory consolidation in scopolamine-induced amnesic mice. Mol Neurobiol 56:6669–6672. https://doi.org/10.1007/s12035-019-01703-9
Penner MR, Roth TL, Chawla MK, Hoang LT, Roth ED, Lubin FD, Sweatt JD, Worley PF et al (2011) Age-related changes in arc transcription and DNA methylation within the hippocampus. Neurobiol Aging 32:2198–2210. https://doi.org/10.1016/j.neurobiolaging.2010.01.009
Singh P, Thakur MK (2018) Histone deacetylase 2 inhibition attenuates downregulation of hippocampal plasticity gene expression during aging. Mol Neurobiol 55:2432–2442. https://doi.org/10.1007/s12035-017-0490-x
Ding X, Liu S, Tian M, Zhang W, Zhu T, Li D et al (2017) Activity-induced histone modifications govern Neurexin-1 mRNA splicing and memory preservation. Nat Neurosci 20:690–699. https://doi.org/10.1038/nn.4536
Kushwaha A, Thakur MK (2020) Increase in hippocampal histone H3K9me3 is negatively correlated with memory in old male mice. Biogerontology 21(2):175–189. https://doi.org/10.1007/s10522-019-09850-1
Snigdha S, Prieto GA, Petrosyan A, Loertscher BM, Dieskau AP, Overman LE, Cotman CW (2016) H3K9me3 inhibition improves memory, promotes spine formation, and increases BDNF levels in the aged hippocampus. J Neurosci 36:3611–3622. https://doi.org/10.1523/JNEUROSCI.2693-15.2016
Cetin A, Komai S, Eliava M, Seeburg PH, Osten P (2007) Stereotaxic gene delivery in the rodent brain. Nat Protoc 1:3166–3173. https://doi.org/10.1038/nprot.2006.450
Baghel MS, Thakur MK (2017) Differential proteome profiling in the hippocampus of amnesic mice. Hippocampus 27:845–859. https://doi.org/10.1002/hipo.22735
Srivas S, Thakur MK (2018) Transcriptional co-repressor SIN3A silencing rescues decline in memory consolidation during scopolamine-induced amnesia. J Neurochem 21:204–216. https://doi.org/10.1111/jnc.14320
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein dye binding. Anal Biochem 72:248–254. https://doi.org/10.1006/abio.1976.9999
Guan J-S, Haggarty SJ, Giacometti E, Dannenberg J-H, Joseph N, Gao J, Nieland TJF, Zhou Y et al (2009) HDAC2 negatively regulates memory formation and synaptic plasticity. Nature 459:55–60. https://doi.org/10.1038/nature07925
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2DDCT method. Methods 25:402–408. https://doi.org/10.1006/meth.2001.1262
Schmittgen TD, Livak KJ (2008) Analyzing real-time PCR data by the comparative C (T) method. Nat Protoc 3:1101–1108. https://doi.org/10.1038/nprot.2008.73
Borlikova GG, Trejo M, Mably AJ, Mc Donald JM, Sala Frigerio C, Regan CM, Murphy KJ, Masliah E et al (2013) Alzheimer brain-derived amyloid β-protein impairs synaptic remodeling and memory consolidation. Neurobiol Aging 34:1315–1327. https://doi.org/10.1016/j.neurobiolaging.2012.10.028
Singh P, Thakur MK (2014) Reduced recognition memory is correlated with decrease in DNA methyltransferase1 and increase in histone deacetylase2 protein expression in old male mice. Biogerontology 15:339–346. https://doi.org/10.1007/s10522-014-9504-5
Kumar D, Thakur MK (2015) Age-related expression of Neurexin1 and Neuroligin3 is correlated with presynaptic density in the cerebral cortex and hippocampus of male mice. Age 37(2):17. https://doi.org/10.1007/s11357-015-9752-6
Kumari A, Thakur MK (2014) Age-dependent decline of nogo-a protein in the mouse cerebrum. Cell Mol Neurobiol 34:1131–1141. https://doi.org/10.1007/s10571-014-0088-z
Day JJ, Sweatt JD (2010) DNA methylation and memory formation. Nat Neurosci 13:1319–1323. https://doi.org/10.1038/nn.2666
Huang PH, Chen CH, Chou CC et al (2011) Histone deacetylase inhibitors stimulate histone H3 lysine 4 methylation in part via transcriptional repression of histone H3 lysine 4 demethylases. Mol Pharmacol 79:197–206. https://doi.org/10.1124/mol.110.067702
Lee MG, Wynder C, Bochar DA et al (2006) Functional interplay between histone demethylase and deacetylase enzymes. Mol Cell Biol 26:6395–6402. https://doi.org/10.1128/MCB.00723-06
Nightingale KP, Gendreizig S, White DA et al (2007) Cross-talk between histone modifications in response to histone deacetylase inhibitors, MLL4 links histone H3 acetylation and histone H3K4 methylation. J Biol Chem 282:4408–4416. https://doi.org/10.1074/jbc.M606773200
Fuks F, Hurd PJ, Deplus R et al (2003) The DNA methyltransferases associate with HP1 and the SUV39H1 histone methyltransferase. Nucleic Acids Res 31:2305–2312. https://doi.org/10.1093/nar/gkg332
Fujita N, Watanabe S, Ichimura T, Tsuruzoe S, Shinkai Y, Tachibana M, Chiba T, Nakao M (2003) Methyl-CpG binding domain 1 (MBD1) interacts with the Suv39h1-HP1 heterochromatic complex for DNA methylation-based transcriptional repression. J Biol Chem 278:24132–24138. https://doi.org/10.1074/jbc.M302283200
Stewart MD, Li J, Wong J (2005) Relationship between histone H3 lysine 9 methylation, transcription repression, and heterochromatin protein 1 recruitment. Mol Cell Biol 25:2525–2538. https://doi.org/10.1128/MCB.25.7.2525-2538.2005
Davis S, Bozon B, Laroche S (2003) How necessary is the activation of the immediate early gene zif268 in synaptic plasticity and learning? Behav Brain Res 142:17–30. https://doi.org/10.1016/s0166-4328(02)00421-7
Miyashita T, Kubik S, Lewandowski G, Guzowski JF (2008) Networks of neurons, networks of genes: an integrated view of memory consolidation. Neurobiol Learn Mem 89:269–284. https://doi.org/10.1016/j.nlm.2007.08.012
Abel T, Lattal KM (2001) Molecular mechanisms of memory acquisition, consolidation and retrieval. Curr Opin Neurobiol 11:180–187. https://doi.org/10.1016/s0959-4388(00)00194-x
Chen QN, Ding XL, Guo XX, Zhou G, Guan JS (2022) Suv39h1 regulates memory stability by inhibiting the expression of Shank1 in hippocampal newborn neurons. Eur J Neurosci 55:1424–1441. https://doi.org/10.1111/ejn.15626
Vaute O, Nicolas E, Vandel L, Trouche D (2002) Functional and physical interaction between the histone methyl transferase Suv39H1 and histone deacetylases. Nucleic Acids Res 30:475–481. https://doi.org/10.1093/nar/30.2.475
Alland L, David G, Shen-Li H, Potes J, Muhle R, Lee HC et al (2002) Identification of mammalian Sds3 as an integral component of the Sin3/histone deacetylase corepressor complex. Mol Cell Biol 22:2743–2750. https://doi.org/10.1128/MCB.22.8.2743-2750.2002
Moser MA, Hagelkruys A, Seiser C (2014) Transcription and beyond: the role of mammalian class I lysine deacetylases. Chromosoma 123:67–78. https://doi.org/10.1007/s00412-013-0441-x
Zhang Y, Ng HH, Erdjument-Bromage H, Tempst P, Bird A, Reinberg D (1999) Analysis of the NuRD subunits reveals a histone deacetylase core complex and a connection with DNA methylation. Genes Dev 13:1924–1935. https://doi.org/10.1101/gad.13.15.1924
Schoch H, Abel T (2014) Transcriptional co-repressors and memory storage. Neuropharmacology 80:53–60. https://doi.org/10.1016/j.neuropharm.2014.01.003
Acknowledgments
The authors acknowledge the use of a real-time PCR facility at the Interdisciplinary School of Life Sciences (ISLS), Banaras Hindu University. We thank the Council of Scientific and Industrial Research (CSIR), India, for a senior research fellowship (AK), and the National Academy of Sciences, India, for a senior scientist fellowship (MKT).
Funding
The authors disclose receipt of the following financial support for the research, authorship, and publication of this article: Department of Science and Technology (EMR/2015/002178), University Grants Commission (F.18-1/2011, BSR) and Indian Council of Medical Research (5/4-5/153/Neuro/2015-NCD-I), Government of India.
Author information
Authors and Affiliations
Contributions
MKT initiated this project. MKT and AK designed the experiments. All authors contributed to and have approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
Our study followed the guidelines of the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA) and all experimental procedures were approved by the Institutional Animal Ethical Committee, Banaras Hindu University, Varanasi.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kushwaha, A., Thakur, M.K. Suv39h1 Silencing Recovers Memory Decline in Scopolamine-Induced Amnesic Mouse Model. Mol Neurobiol 61, 487–497 (2024). https://doi.org/10.1007/s12035-023-03570-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-023-03570-x