Abstract
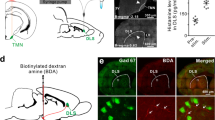
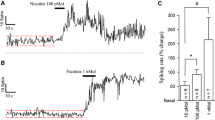
The central histaminergic nervous system, originating from the tuberomammillary nucleus (TMN) of the hypothalamus, widely innervates almost the whole brain, including the basal ganglia. Intriguingly, the histaminergic system is altered in parkinsonian patients. Yet, little is known about the effect and mechanisms of histamine on different types of neurons in the basal ganglia circuitry. Here, by using anterograde tracing, immunostaining, patch clamp recording, and single-cell qPCR techniques, we investigate the histaminergic afferents in the striatum, the major input structure of the basal ganglia, as well as the effect of histamine on the striatal GABAergic medium spiny projection neurons (MSNs). We report a direct histaminergic projection from the hypothalamic TMN to the striatum in rats. Furthermore, histamine exerts a strong postsynaptic excitatory effect on both dopamine D1 and D2 receptor-expressing MSNs. The concentration-response curves and the EC50 values for histamine on these two types of MSNs are similar. In addition, dopamine D1 and D2 receptor-expressing MSNs co-express histamine H1 and H2 receptor mRNAs. Both histamine H1 and H2 receptors are co-localized on dopamine D1 and D2 receptor-expressing MSNs and co-mediate the histamine-induced excitation on the two types of neurons. These results suggest that the histaminergic afferent inputs in the striatum may modulate both dopamine D1 and D2 receptor-expressing MSNs by activation of postsynaptic histamine H1 and H2 receptors and thus serve as an important extrastriatal modulator for biasing the direct and indirect pathways to actively regulate functions of the basal ganglia and participate in the pathogenesis and pathophysiology of basal ganglia diseases.





Similar content being viewed by others
References
Haas H, Panula P (2003) The role of histamine and the tuberomamillary nucleus in the nervous system. Nat Rev Neurosci 4(2):121–130. https://doi.org/10.1038/nrn1034
Haas HL, Sergeeva OA, Selbach O (2008) Histamine in the nervous system. Physiol Rev 88(3):1183–1241. https://doi.org/10.1152/physrev.00043.2007
Fiorentini C, Savoia P, Savoldi D, Barbon A, Missale C (2013) Persistent activation of the D1R/Shp-2/Erk1/2 pathway in l-DOPA-induced dyskinesia in the 6-hydroxy-dopamine rat model of Parkinson's disease. Neurobiol Dis 54:339–348. https://doi.org/10.1016/j.nbd.2013.01.005
Bolam JP, Ellender TJ (2015) Histamine and the striatum. Neuropharmacology 106:74–84. https://doi.org/10.1016/j.neuropharm.2015.08.013
Panula P, Chazot PL, Cowart M, Gutzmer R, Leurs R, Liu WL, Stark H, Thurmond RL et al (2015) International union of basic and clinical pharmacology. XCVIII. Histamine receptors. Pharmacol Rev 67(3):601–655. https://doi.org/10.1124/pr.114.010249
Rinne JO, Anichtchik OV, Eriksson KS, Kaslin J, Tuomisto L, Kalimo H, Roytta M, Panula P (2002) Increased brain histamine levels in Parkinson’s disease but not in multiple system atrophy. J Neurochem 81(5):954–960. https://doi.org/10.1046/j.1471-4159.2002.00871.x
Anichtchik OV, Peitsaro N, Rinne JO, Kalimo H, Panula P (2001) Distribution and modulation of histamine H(3) receptors in basal ganglia and frontal cortex of healthy controls and patients with Parkinson’s disease. Neurobiol Dis 8(4):707–716. https://doi.org/10.1006/nbdi.2001.0413
van Wamelen DJ, Shan L, Aziz NA, Anink JJ, Bao AM, Roos RA, Swaab DF (2011) Functional increase of brain histaminergic signaling in Huntington’s disease. Brain Pathol 21(4):419–427. https://doi.org/10.1111/j.1750-3639.2010.00465.x
Chen K, Wang JJ, Yung WH, Chan YS, Chow BK (2005) Excitatory effect of histamine on neuronal activity of rat globus pallidus by activation of H2 receptors in vitro. Neurosci Res 53(3):288–297. https://doi.org/10.1016/j.neures.2005.07.008
Korotkova TM, Haas HL, Brown RE (2002) Histamine excites GABAergic cells in the rat substantia nigra and ventral tegmental area in vitro. Neurosci Lett 320(3):133–136. https://doi.org/10.1016/S0304-3940(02)00050-2
Graybiel AM, Aosaki T, Flaherty AW, Kimura M (1994) The basal ganglia and adaptive motor control. Science 265(5180):1826–1831. https://doi.org/10.1126/science.8091209
Grillner S, Hellgren J, Menard A, Saitoh K, Wikstrom MA (2005) Mechanisms for selection of basic motor programs—roles for the striatum and pallidum. Trends Neurosci 28(7):364–370. https://doi.org/10.1016/j.tins.2005.05.004
Yin HH, Knowlton BJ (2006) The role of the basal ganglia in habit formation. Nat Rev Neurosci 7(6):464–476. https://doi.org/10.1038/nrn1919
Canales JJ (2005) Stimulant-induced adaptations in neostriatal matrix and striosome systems: transiting from instrumental responding to habitual behavior in drug addiction. Neurobiol Learn Mem 83(2):93–103. https://doi.org/10.1016/j.nlm.2004.10.006
Kreitzer AC, Malenka RC (2007) Endocannabinoid-mediated rescue of striatal LTD and motor deficits in Parkinson’s disease models. Nature 445(7128):643–647. https://doi.org/10.1038/nature05506
Luscher C, Huber KM (2010) Group 1 mGluR-dependent synaptic long-term depression: mechanisms and implications for circuitry and disease. Neuron 65(4):445–459. https://doi.org/10.1016/j.neuron.2010.01.016
Gerfen CR, Engber TM, Mahan LC, Susel Z, Chase TN, Monsma FJ Jr, Sibley DR (1990) D1 and D2 dopamine receptor-regulated gene expression of striatonigral and striatopallidal neurons. Science 250(4986):1429–1432. https://doi.org/10.1126/science.2147780
Surmeier DJ, Ding J, Day M, Wang Z, Shen W (2007) D1 and D2 dopamine-receptor modulation of striatal glutamatergic signaling in striatal medium spiny neurons. Trends Neurosci 30(5):228–235. https://doi.org/10.1016/j.tins.2007.03.008
Vizuete ML, Traiffort E, Bouthenet ML, Ruat M, Souil E, Tardivel-Lacombe J, Schwartz JC (1997) Detailed mapping of the histamine H2 receptor and its gene transcripts in guinea-pig brain. Neuroscience 80(2):321–343. https://doi.org/10.1016/S0306-4522(97)00010-9
Pillot C, Heron A, Cochois V, Tardivel-Lacombe J, Ligneau X, Schwartz JC, Arrang JM (2002) A detailed mapping of the histamine H(3) receptor and its gene transcripts in rat brain. Neuroscience 114(1):173–193. https://doi.org/10.1016/S0306-4522(02)00135-5
Ellender TJ, Huerta-Ocampo I, Deisseroth K, Capogna M, Bolam JP (2011) Differential modulation of excitatory and inhibitory striatal synaptic transmission by histamine. J Neurosci 31(43):15340–15351. https://doi.org/10.1523/JNEUROSCI.3144-11.2011
Doreulee N, Yanovsky Y, Flagmeyer I, Stevens DR, Haas HL, Brown RE (2001) Histamine H(3) receptors depress synaptic transmission in the corticostriatal pathway. Neuropharmacology 40(1):106–113. https://doi.org/10.1016/S0028-3908(00)00101-5
Colwell CS, Levine MS (1997) Histamine modulates NMDA-dependent swelling in the neostriatum. Brain Res 766(1–2):205–212. https://doi.org/10.1016/S0006-8993(97)00557-X
McGrath JC, Drummond GB, McLachlan EM, Kilkenny C, Wainwright CL (2010) Guidelines for reporting experiments involving animals: the ARRIVE guidelines. Br J Pharmacol 160(7):1573–1576. https://doi.org/10.1111/j.1476-5381.2010.00873.x
Paxinos G, Watson C (2014) The rat atlas in stereotaxic coordinates, 7th edn. Academic Press, San Diego
Li B, Zhuang QX, Gao HR, Wang JJ, Zhu JN (2017) Medial cerebellar nucleus projects to feeding-related neurons in the ventromedial hypothalamic nucleus in rats. Brain Struct Funct 222(2):957–971. https://doi.org/10.1007/s00429-016-1257-2
Wang Y, Chen ZP, Zhuang QX, Zhang XY, Li HZ, Wang JJ, Zhu JN (2017) Role of corticotropin-releasing factor in cerebellar motor control and ataxia. Curr Biol 27(17):2661–2669 e2665. https://doi.org/10.1016/j.cub.2017.07.035
Zhang J, Li B, Yu L, He YC, Li HZ, Zhu JN, Wang JJ (2011) A role for orexin in central vestibular motor control. Neuron 69(4):793–804. https://doi.org/10.1016/j.neuron.2011.01.026
Zhang J, Zhuang QX, Li B, Wu GY, Yung WH, Zhu JN, Wang JJ (2016) Selective modulation of histaminergic inputs on projection neurons of cerebellum rapidly promotes motor coordination via HCN channels. Mol Neurobiol 53(2):1386–1401. https://doi.org/10.1007/s12035-015-9096-3
Zhang XY, Yu L, Zhuang QX, Peng SY, Zhu JN, Wang JJ (2013) Postsynaptic mechanisms underlying the excitatory action of histamine on medial vestibular nucleus neurons in rats. Br J Pharmacol 170(1):156–169. https://doi.org/10.1111/bph.12256
Koos T, Tepper JM (1999) Inhibitory control of neostriatal projection neurons by GABAergic interneurons. Nat Neurosci 2(5):467–472. https://doi.org/10.1038/8138
Chuhma N, Tanaka KF, Hen R, Rayport S (2011) Functional connectome of the striatal medium spiny neuron. J Neurosci 31(4):1183–1192. https://doi.org/10.1523/JNEUROSCI.3833-10.2011
Brown RE, Stevens DR, Haas HL (2001) The physiology of brain histamine. Prog Neurobiol 63(6):637–672. https://doi.org/10.1016/S0301-0082(00)00039-3
Zhu JN, Yung WH, Kwok-Chong Chow B, Chan YS, Wang JJ (2006) The cerebellar-hypothalamic circuits: potential pathways underlying cerebellar involvement in somatic-visceral integration. Brain Res Rev 52(1):93–106. https://doi.org/10.1016/j.brainresrev.2006.01.003
Chazot PL (2013) Histamine pharmacology: four years on. Br J Pharmacol 170(1):1–3. https://doi.org/10.1111/bph.12319
Williams RH, Chee MJ, Kroeger D, Ferrari LL, Maratos-Flier E, Scammell TE, Arrigoni E (2014) Optogenetic-mediated release of histamine reveals distal and autoregulatory mechanisms for controlling arousal. J Neurosci 34(17):6023–6029. https://doi.org/10.1523/JNEUROSCI.4838-13.2014
Kim YS, Kim YB, Kim WB, Yoon BE, Shen FY, Lee SW, Soong TW, Han HC et al (2015) Histamine resets the circadian clock in the suprachiasmatic nucleus through the H1R-CaV 1.3-RyR pathway in the mouse. Eur J Neurosci 42(7):2467–2477. https://doi.org/10.1111/ejn.13030
Eban-Rothschild A, Giardino WJ, de Lecea L (2017) To sleep or not to sleep: neuronal and ecological insights. Curr Opin Neurobiol 44:132–138. https://doi.org/10.1016/j.conb.2017.04.010
Sallmen T, Beckman AL, Stanton TL, Eriksson KS, Tarhanen J, Tuomisto L, Panula P (1999) Major changes in the brain histamine system of the ground squirrel Citellus lateralis during hibernation. J Neurosci 19(5):1824–1835
Anichtchik OV, Rinne JO, Kalimo H, Panula P (2000) An altered histaminergic innervation of the substantia nigra in Parkinson’s disease. Exp Neurol 163(1):20–30. https://doi.org/10.1006/exnr.2000.7362
Lian J, De Santis M, He M, Deng C (2015) Risperidone-induced weight gain and reduced locomotor activity in juvenile female rats: the role of histaminergic and NPY pathways. Pharmacol Res 95-96:20–26. https://doi.org/10.1016/j.phrs.2015.03.004
Panula P, Nuutinen S (2013) The histaminergic network in the brain: basic organization and role in disease. Nat Rev Neurosci 14(7):472–487. https://doi.org/10.1038/nrn3526
Gomez-Ramirez J, Johnston TH, Visanji NP, Fox SH, Brotchie JM (2006) Histamine H3 receptor agonists reduce L-dopa-induced chorea, but not dystonia, in the MPTP-lesioned nonhuman primate model of Parkinson’s disease. Mov Disord 21(6):839–846. https://doi.org/10.1002/mds.20828
Nowak P, Bortel A, Dabrowska J, Biedka I, Slomian G, Roczniak W, Kostrzewa RM, Brus R (2008) Histamine H(3) receptor ligands modulate L-dopa-evoked behavioral responses and L-dopa derived extracellular dopamine in dopamine-denervated rat striatum. Neurotox Res 13(3–4):231–240
Liu CQ, Hu DN, Liu FX, Chen Z, Luo JH (2008) Apomorphine-induced turning behavior in 6-hydroxydopamine lesioned rats is increased by histidine and decreased by histidine decarboxylase, histamine H1 and H2 receptor antagonists, and an H3 receptor agonist. Pharmacol Biochem Behav 90(3):325–330. https://doi.org/10.1016/j.pbb.2008.03.010
Ferrada C, Ferre S, Casado V, Cortes A, Justinova Z, Barnes C, Canela EI, Goldberg SR et al (2008) Interactions between histamine H-3 and dopamine D-2 receptors and the implications for striatal function. Neuropharmacology 55(2):190–197. https://doi.org/10.1016/j.neuropharm.2008.05.008
González-Sepúlveda M, Rosell S, Hoffmann HM, del Mar Castillo-Ruiz M, Mignon V, Moreno-Delgado D, Vignes M, Díaz J et al (2013) Cellular distribution of the histamine H3 receptor in the basal ganglia: Functional modulation of dopamine and glutamate neurotransmission. Basal Ganglia 3(2):109–121. https://doi.org/10.1016/j.baga.2012.12.001
Calabresi P, Picconi B, Tozzi A, Ghiglieri V, Di Filippo M (2014) Direct and indirect pathways of basal ganglia: a critical reappraisal. Nat Neurosci 17(8):1022–1030. https://doi.org/10.1038/nn.3743
Wichmann T, Delong MR (2006) Deep brain stimulation for neurologic and neuropsychiatric disorders. Neuron 52(1):197–204. https://doi.org/10.1016/j.neuron.2006.09.022
Ellenbroek BA, Ghiabi B (2014) The other side of the histamine H3 receptor. Trends Neurosci 37(4):191–199. https://doi.org/10.1016/j.tins.2014.02.007
Shan L, Dauvilliers Y, Siegel JM (2015) Interactions of the histamine and hypocretin systems in CNS disorders. Nat Rev Neurol 11(7):401–413. https://doi.org/10.1038/nrneurol.2015.99
Funding
This work was supported by the National Natural Science Foundation of China (grant numbers 31330033, 81671107, 31471112, 31771143, 31500848, 91332124, and NSFC/RGC Joint Research Scheme 31461163001); the Ministry of Education, China (SRFDP/RGC ERG grant 20130091140003, and Fundamental Research Funds for the Central Universities 020814380004); and the Natural Science Foundation of Jiangsu Province, China (grant numbers BK20151384 and BK20140599).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
All experiments were carried out in accordance with the US National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publication 85–23, revised 2011) and complied with the ARRIVE guidelines.
Conflict of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Zhuang, QX., Xu, HT., Lu, XJ. et al. Histamine Excites Striatal Dopamine D1 and D2 Receptor-Expressing Neurons via Postsynaptic H1 and H2 Receptors. Mol Neurobiol 55, 8059–8070 (2018). https://doi.org/10.1007/s12035-018-0976-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-018-0976-1