Abstract
Recent studies show that microRNA-34 (miR-34) family is critical in the regulation of stress response also suggesting that it may contribute to the individual responsiveness to stress. We have recently demonstrated that mice carrying a genetic deletion of all miR-34 isoforms (triple knockout, TKO) lack the stress-induced serotonin (5-HT) and GABA release in the medial prefrontal cortex (mpFC) and basolateral amygdala (BLA), respectively. Here, we evaluated if the absence of miR-34 was also able to modify the stress-coping strategy in the forced swimming test. We found that the blunted neurochemical response to stress was associated with lower levels of immobility (index of active coping behavior) in TKO compared to WT mice. Interestingly, among the brain regions mostly involved in the stress-related behaviors, the miR-34 displayed the strongest expression in the dorsal raphe nuclei (DRN) of wild-type (WT) mice. In the DRN, the corticotropin-releasing factor receptors (CRFR) 1 and 2, contribute to determine the stress-coping style and the CRFR1 is a target of miR-34. Thus, we hypothesized that the miR-34-dependent modulation of CRFR1 expression may be involved in the DRN regulation of stress-coping strategies. In line with this hypothesis, we found increased CRFR1 levels in the DNR of TKO compared to WT mice. Moreover, infusion of CRFR1 antagonist in the DRN of TKO mice reverted their behavioral and neurochemical phenotype. We propose that miR-34 modulate the mpFC 5-HT/BLA GABA response to stress acting on CRFR1 in the DRN and that this mechanism could contribute to determine individual stress-coping strategy.







Similar content being viewed by others
Change history
10 December 2019
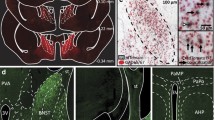
The original version of this article unfortunately contained a mistake in Figure 3. The drawing superimposed on photomicrographs to identify the region of Dorsal raph�� Nuclei was inappropriately positioned. The corrected figure is given below.
References
Ursin H, Olff M (1995) Aggression, defense, and coping in humans. Aggress Behav 21(1):13–19. https://doi.org/10.1002/1098-2337(1995)21:1<13::AID-AB2480210104>3.0.CO;2-Z
Koolhaas JM, de Boer SF, Buwalda B, van Reenen K (2007) Individual variation in coping with stress: a multidimensional approach of ultimate and proximate mechanisms. Brain Behav Evol 70(4):218–226. https://doi.org/10.1159/000105485
Wechsler B (1995) Coping and coping strategies: a behavioural view. Appl Anim Behaviour Sci 43(2):123–134. https://doi.org/10.1016/0168-1591(95)00557-9
Lazarus R (1966) Psychosocial stress and the coping process. McGraw-Hill, New York
McEwen BS (2007) Physiology and neurobiology of stress and adaptation: central role of the brain. Physiol Rev 87(3):873–904. https://doi.org/10.1152/physrev.00041.2006
Maier SF, Watkins LR (2005) Stressor controllability and learned helplessness: the roles of the dorsal raphe nucleus, serotonin, and corticotropin-releasing factor. Neurosci Biobehav Rev 29(4-5):829–841. https://doi.org/10.1016/j.neubiorev.2005.03.021
Taylor SE, Stanton AL (2007) Coping resources, coping processes, and mental health. Annu Rev Clin Psychol 3(1):377–401. https://doi.org/10.1146/annurev.clinpsy.3.022806.091520
Malan-Müller S, Hemmings SM, Seedat S (2013) Big effects of small RNAs: a review of microRNAs in anxiety. Mol Neurobiol 47(2):726–739. https://doi.org/10.1007/s12035-012-8374-6
Issler O, Chen A (2015) Determining the role of microRNAs in psychiatric disorders. Nat Rev Neurosci 16(4):201–212. https://doi.org/10.1038/nrn3879
Schouten M, Aschrafi A, Bielefeld P, Doxakis E, Fitzsimons CP (2013) MicroRNAs and the regulation of neuronal plasticity under stress conditions. Neuroscience 241:188–205. https://doi.org/10.1016/j.neuroscience.2013.02.065.
O’Connor RM, Dinan TG, Cryan JF (2012) Little things on which happiness depends: microRNAs as novel therapeutic targets for the treatment of anxiety and depression. Mol Psychiatry 17(4):359–376. https://doi.org/10.1038/mp.2011.162
Zurawek D, Kusmider M, Faron-Gorecka A, Gruca P, Pabian P, Solich J, Kolasa M, Papp M et al (2016) Reciprocal MicroRNA expression in mesocortical circuit and its interplay with serotonin transporter define resilient rats in the chronic mild stress. Mol Neurobiol 54(8):5741–5751. https://doi.org/10.1007/s12035-016-0107-9
Cohen JL, Ata AE, Jackson NL, Rahn EJ, Ramaker RC, Cooper S, Kerman IA, Clinton SM (2017) Differential stress induced c-Fos expression and identification of region-specific miRNA-mRNA networks in the dorsal raphe and amygdala of high-responder/low-responder rats. Behav Brain Res 319:110–123. https://doi.org/10.1016/j.bbr.2016.11.015
Chen RJ, Kelly G, Sengupta A, Heydendael W, Nicholas B, Beltrami S, Luz S, Peixoto L et al (2015) MicroRNAs as biomarkers of resilience or vulnerability to stress. Neuroscience 305:36–48. https://doi.org/10.1016/j.neuroscience.2015.07.045
Jauhari A, Singh T, Singh P, Parmar D, Yadav S (2017) Regulation of miR-34 family in neuronal development. Mol Neurobiol. https://doi.org/10.1007/s12035-016-0359-4
Concepcion CP, Han YC, Mu P, Bonetti C, Yao E, D’Andrea A, Vidigal JA, Maughan WP et al (2012) Intact p53-dependent responses in miR-34-deficient mice. PLoS Genet 8(7):e1002797. https://doi.org/10.1371/journal.pgen.1002797
Bernardo BC, Gao XM, Winbanks CE, Boey EJ, Tham YK, Kiriazis H, Gregorevic P, Obad S et al (2012) Therapeutic inhibition of the miR-34 family attenuates pathological cardiac remodeling and improves heart function. Proc Natl Acad Sci USA 109(43):17615–17620. https://doi.org/10.1073/pnas.1206432109
Zovoilis A, Agbemenyah HY, Agis-Balboa RC, Stilling RM, Edbauer D, Rao P, Farinelli L, Delalle I et al (2011) microRNA-34c is a novel target to treat dementias. EMBO J 30(20):4299–4308. https://doi.org/10.1038/emboj.2011.327
Haramati S, Navon I, Issler O, Ezra-Nevo G, Gil S, Zwang R, Hornstein E, Chen A (2011) MicroRNA as repressors of stress-induced anxiety: the case of amygdalar miR-34. J Neurosci 31(40):14191e14203–14191e14203. https://doi.org/10.1523/JNEUROSCI.1673-11.2011
Andolina D, Di Segni M, Bisicchia E, D’Alessandro F, Cestari V, Ventura A, Concepcion C, Puglisi-Allegra S et al (2016) Effects of lack of microRNA-34 on the neural circuitry underlying the stress response and anxiety. Neuropharmacology 107:305–316. https://doi.org/10.1016/j.neuropharm.2016.03.044
Bavamian S, Mellios N, Lalonde J, Fass DM, Wang J, Sheridan SD, Madison JM, Zhou F et al (2015) Dysregulation of miR-34a links neuronal development to genetic risk factors for bipolar disorder. Mol Psychiatry 20(5):573e584–573e584. https://doi.org/10.1038/mp.2014.176
Agostini M, Tucci P, Steinert JR, Shalom-Feuerstein R, Rouleau M, Aberdam D, Forsythe ID, Young KW et al (2011) microRNA-34a regulates neurite outgrowth, spinal morphology, and function. Proc Natl Acad Sci U S A 108(52):21099–21104. https://doi.org/10.1073/pnas.1112063108
Zhou R, Yuan P, Wang Y, Hunsberger JG, Elkahloun A, Wei Y, Damschroder-Williams P, Du J et al (2009) Evidence for selective microRNAs and their effectors as common long-term targets for the actions of mood stabilizers. Neuropsychopharmacology 34(6):1395e1405–1395e1405. https://doi.org/10.1038/npp.2008.131
Bocchio-Chiavetto L, Maffioletti E, Bettinsoli P, Giovannini C, Bignotti S, Tardito D, Corrada D, Milanesi L et al (2013) Blood microRNA changes in depressed patients during antidepressant treatment. Eur J Neuropsychopharmacol 23(7):602e611–602e611. https://doi.org/10.1016/j.euroneuro.2012.06.013
Xu C, Yang C, Zhang A, Xu Y, Li X, Liu Z, Liu S, Sun N et al (2017) The interaction of miR-34b/c polymorphisms and negative life events increases susceptibility to major depressive disorder in Han Chinese population. Neurosci Lett 651:65–71. https://doi.org/10.1016/j.neulet.2017.04.061
Dias BG, Goodman JV, Ahluwalia R, Easton AE, Andero R, Ressler KJ (2014) Amygdala-dependent fear memory consolidation via miR-34a and notch signaling. Neuron 83(4):906e918–906e918. https://doi.org/10.1016/j.neuron.2014.07.019
Waselus M, Nazzaro C, Valentino RJ, Van Bockstaele EJ (2009) Stress-induced redistribution of corticotropin-releasing factor receptor subtypes in the dorsal raphe nucleus. Biol Psychiatry 66(1):76–83. https://doi.org/10.1016/j.biopsych.2009.02.014
Wood SK, Zhang XY, Reyes BA, Lee CS, Van Bockstaele EJ, Valentino RJ (2013) Cellular adaptations of dorsal raphe serotonin neurons associated with the development of active coping in response to social stress. Biol Psychiatry 73(11):1087–1094. https://doi.org/10.1016/j.biopsych.2013.01.026
Waselus M, Valentino RJ, Van Bockstaele EJ (2011) Collateralized dorsal raphe nucleus projections: a mechanism for the integration of diverse functions during stress. J Chem Neuroanat 41(4):266–280. https://doi.org/10.1016/j.jchemneu.2011.05.011
Snyder KP, Hill-Smith TE, Lucki I, Valentino RJ (2015) Corticotropin-releasing factor in the rat dorsal raphe nucleus promotes different forms of behavioral flexibility depending on social stress history. Neuropsychopharmacology 40(11):2517–2525. https://doi.org/10.1038/npp.2015.98
Andolina D, Maran D, Valzania A, Conversi D, Puglisi-Allegra S (2013) Prefrontal/amygdalar system determines stress coping behavior through 5-HT/GABA connection. Neuropsychopharmacology 38(10):2057–2067. https://doi.org/10.1038/npp.2013.107
Andolina D, Maran D, Viscomi MT, Puglisi-Allegra S (2014) Strain-dependent variations in stress coping behavior are mediated by a 5-HT/GABA interaction within the prefrontal corticolimbic system. Int J Neuropsychopharmacol 18(3):pyu074. https://doi.org/10.1093/ijnp/pyu074
Molendijk ML, de Kloet ER (2015) Immobility in the forced swim test is adaptive and does not reflect depression. Psychoneuroendocrinology 62:389–391. https://doi.org/10.1016/j.psyneuen.2015.08.028
de Kloet ER, Molendijk ML (2016) Coping with the forced swim stressor: towards understanding an adaptive mechanism. Neural Plast 2016:6503162–6503113. https://doi.org/10.1155/2016/6503162
Franklin KBJ, Paxinos G (2004) The mouse brain: in stereotaxic coordinates, 2nd edn. Academic, San Diego
Schmittgen TD, Livak KJ (2008) Analyzing real-time PCR data by the comparative C (T) method. Nat Protoc 3(6):1101–1108. https://doi.org/10.1038/nprot.2008.73
Howerton AR, Roland AV, Fluharty JM, Marshall A, Chen A, Daniels D, Beck SG, Bale TL (2014) Sex differences in corticotropin-releasing factor receptor-1 action within the dorsal raphe nucleus in stress responsivity. Biol Psychiatry 75(11):873–883. https://doi.org/10.1016/j.biopsych.2013.10.013
Sutton SW, Behan DP, Lahrichi SL, Kaiser R, Corrigan A, Lowry P, Potter E et al (1995) Ligand requirements of the human corticotropin-releasing factor-binding protein. Endocrinology 136(3):1097–1102. https://doi.org/10.1210/endo.136.3.7867564
Takahashi A, Shimamoto A, Boyson CO, DeBold JF, Miczek KA (2010) GABA(B) receptor modulation of serotonin neurons in the dorsal raphe nucleus and escalation of aggression in mice. J Neurosci 30(35):11771–11780. https://doi.org/10.1523/JNEUROSCI.1814-10.2010
Di Chiara G, Tanda G, Frau R, Carboni E (1993) On the preferential release of dopamine in the nucleus accumbens by amphetamine: further evidence obtained by vertically implanted concentric dialysis probes. Psychopharmacology (Berlin) 112:398–402
Rea K, Cremers TI, Westerink BH (2005) HPLC conditions are critical for the detection of GABA by microdialysis. J Neurochem 94(3):672679–672679. https://doi.org/10.1111/j.1471-4159.2005.03218.x
Meister G, Landthaler M, Patkaniowska A, Dorsett Y, Teng G, Tuschl T (2004) Human argonaute2 mediates RNA cleavage targeted by miRNAs and siRNAs. Mol Cell 15(2):185–197. https://doi.org/10.1016/j.molcel.2004.07.007
Puglisi-Allegra S, Andolina D (2015) Serotonin and stress coping. Behav Brain Res 277:58–67. https://doi.org/10.1016/j.bbr.2014.07.052
Choi NG, Hegel MT, Sirrianni L, Marinucci ML, Bruce LM (2012) Passive coping response to depressive symptoms among low-income homebound older adults: does it affect depression severity and treatment outcome? Behav Res Ther 50(11):668–674. https://doi.org/10.1016/j.brat.2012.07.003
Bardwell WA, Ancoli-Israel S, Dimsdale JE (2001) Types of coping strategies are associated with increased depressive symptoms in patients with obstructive sleep apnea. Sleep 24(8):905–909. https://doi.org/10.1093/sleep/24.8.905
Patterson TL, Semple SJ, Temoshok LR, Atkinson JH, McCutchan JA, Straits-Troster KA, Chandler JL, Grant I (1993) Depressive symptoms among HIV positive men: life stress, coping and social support. J Appl Biobehav Res 1(1):64–87. https://doi.org/10.1111/j.1751-9861.1993.tb00028.x
Sun N, Lei L, Wang Y, Yang C, Liu Z, Li X, Zhang K (2016) Preliminary comparison of plasma notch-associated microRNA-34b and -34c levels in drug naive, first episode depressed patients and healthy controls. J Affect Disord 194:109–114. https://doi.org/10.1016/j.jad.2016.01.017
Valentino RJ, Lucki I, Van Bockstaele E (2010) Corticotropin-releasing factor in the dorsal raphe nucleus: linking stress coping and addiction. Brain Res 1314:29–37. https://doi.org/10.1016/j.brainres.2009.09.100
Amat J, Baratta MV, Paul E, Bland ST, Watkins LR, Maier SF (2005) Medial prefrontal cortex determines how stressor controllability affects behavior and dorsal raphe nucleus. Nat Neurosci 8(3):365–371. https://doi.org/10.1038/nn1399
Holmes A (2008) Genetic variation in cortico-amygdala serotonin function and risk for stress-related disease. Neurosci Biobehav Rev 32(7):1293–1314. https://doi.org/10.1016/j.neubiorev.2008.03.006
Kirby LG, Rice KC, Valentino RJ (2000) Effects of corticotropin-releasing factor on neuronal activity in the serotonergic dorsal raphe nucleus. Neuropsychopharmacology 22(2):148–162. https://doi.org/10.1016/S0893-133X(99)00093-7
Kirby LG, Freeman-Daniels E, Lemos JC, Nunan JD, Lamy C, Akanwa A, Beck SG (2008) Corticotropin-releasing factor increases GABA synaptic activity and induces inward current in 5-hydroxytryptamine dorsal raphe neurons. J Neurosci 28(48):12927–12937. https://doi.org/10.1523/JNEUROSCI.2887-08.2008
Price ML, Lucki I (2001) Regulation of serotonin release in the lateral septum and striatum by corticotropin-releasing factor. J Neurosci 21(8):2833–2841
Roche M, Commons KG, Peoples A, Valentino RJ (2003) Circuitry underlying regulation of the serotonergic system by swim stress. J Neurosci 23(3):970–977
Waselus M, Valentino RJ, Van Bockstaele EJ (2005) Ultrastructural evidence for a role of gamma-aminobutyric acid in mediating the effects of corticotropin-releasing factor on the rat dorsal raphe serotonin system. J Comp Neurol 482(2):155–165. https://doi.org/10.1002/cne.20360
Hammack SE, Schmid MJ, LoPresti ML, Der-Avakian A, Pellymounter MA, Foster AC, Watkins LR, Maier SF (2003) Corticotropin releasing hormone type 2 receptors in the dorsal raphe nucleus mediate the behavioral consequences of uncontrollable stress. J Neurosci 23(3):1019–1025
Fox JH, Lowry CA (2013) Corticotropin-releasing factor-related peptides, serotonergic systems, and emotional behavior. Front Neurosci 7:169. https://doi.org/10.3389/fnins.2013.00169
Lukkes JL, Forster GL, Renner KJ, Summers CH (2008) Corticotropin-releasing factor 1 and 2 receptors in the dorsal raphé differentially affect serotonin release in the nucleus accumbens. Eur J Pharmacol 578(2-3):185–193. https://doi.org/10.1016/j.ejphar.2007.09.024
Acknowledgements
miR-34 KO mice were kindly provided by Dr. Andrea Ventura (Memorial Sloan Kettering Cancer Center, NY, USA).
Funding
This research was supported by SIR “RBSI14G1HH”, Italian Ministry of Education, Universities and Research (MIUR); and Ateneo 2015, Sapienza University of Rome “C26A15S53E”.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no competing interests.
Electronic supplementary material
Fig. A1
Genotyping protocol (a) and miR-34a and c expression in dorsal raphe nuclei in WT and TKO mice (b) (GIF 56 kb)
Fig. A2
Representative photomicrograph showing the injection sites in the dorsal raphe nuclei (DRN) (GIF 1408 kb)
Fig. A3
Effects of the genotype (WT n = 4; TKO n = 4) on CRFR2 levels in dorsal raphe nuclei (DRN). Detection of CRFR2 and tubulin (used as loading control) (upper line) and relative histogram (lower line). Data are shown as relative ratio ± SE (GIF 16 kb)
Fig. A4
CRFR2 immunoreactivity in the dorsal raphe nuclei (DRN) of WT and TKO mice. Atlas diagrams reprinted from Franklin and Paxinos (Franklin and Paxinos 2004) indicating representative images of the DRN (a). Histogram of densitometric values of CRFR2 immunofluorescence expressed as mean fluorescence of individual cells normalized to total cellular surface (F/A) of WT (n = 7) and TKO (n = 7) mice. Data are reported as means ± SE (b). Triple-595 labeled confocal images of DAPI staining (blue), Serotonin (5-HT) (yellow), CRFR2 (red) plus merged of DRN (c) (GIF 40 kb)
Fig. A5
Effects of dorsal raphe nuclei (DRN) infusion of the artificial cerebrospinal fluid (CSF, (WT n = 4; TKO n = 4)), CRF 50 ng in WT (n = 4) or NBI 35965 4.4 ng in TKO (n = 4) on distance moved (cm) in the locomotor activity test (GIF 8 kb)
Rights and permissions
About this article
Cite this article
Andolina, D., Di Segni, M., Accoto, A. et al. MicroRNA-34 Contributes to the Stress-related Behavior and Affects 5-HT Prefrontal/GABA Amygdalar System through Regulation of Corticotropin-releasing Factor Receptor 1. Mol Neurobiol 55, 7401–7412 (2018). https://doi.org/10.1007/s12035-018-0925-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-018-0925-z