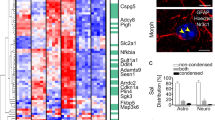
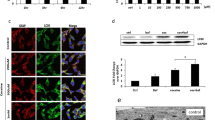
Abstract
A recent study from our lab has revealed a link between morphine-mediated autophagy and synaptic impairment. The current study was aimed at investigating whether morphine-mediated activation of astrocytes involved the ER stress/autophagy axis. Our in vitro findings demonstrated upregulation of GFAP indicating astrocyte activation with a concomitant increase in the production of proinflammatory cytokines in morphine-exposed human astrocytes. Using both pharmacological and gene-silencing approaches, it was demonstrated that morphine-mediated defective autophagy involved upstream activation of ER stress with subsequent downstream astrocyte activation via the μ-opioid receptor (MOR). In vivo validation demonstrated preferential activation of ER stress/autophagy axis in the areas of the brain not associated with pain such as the basal ganglia, frontal cortex, occipital cortex, and the cerebellum of morphine-dependent rhesus macaques, and this correlated with increased astrocyte activation and neuroinflammation. Interventions aimed at blocking either the MOR or ER stress could thus likely be developed as promising therapeutic targets for abrogating morphine-mediated astrocytosis.










Similar content being viewed by others
References
Stefano GB, Ptacek R, Kuzelova H, Kream RM (2012) Endogenous morphine: up-to-date review 2011. Folia Biol 58(2):49–56
Johnson F, Setnik B (2011) Morphine sulfate and naltrexone hydrochloride extended-release capsules: naltrexone release, pharmacodynamics, and tolerability. Pain Physician 14(4):391–406
Ting S, Schug S (2016) The pharmacogenomics of pain management: prospects for personalized medicine. J Pain Res 9:49–56. https://doi.org/10.2147/JPR.S55595
Morizio KM, Baum RA, Dugan A, Martin JE, Bailey AM (2017) Characterization and management of patients with heroin versus nonheroin opioid overdoses: experience at an Academic Medical Center. Pharmacotherapy 37(7):781–790. https://doi.org/10.1002/phar.1902
Friedman H, Eisenstein TK (2004) Neurological basis of drug dependence and its effects on the immune system. J Neuroimmunol 147(1–2):106–108. https://doi.org/10.1016/j.jneuroim.2003.10.022
Roy S, Wang J, Kelschenbach J, Koodie L, Martin J (2006) Modulation of immune function by morphine: implications for susceptibility to infection. J NeuroImmune Pharmacol 1(1):77–89. https://doi.org/10.1007/s11481-005-9009-8
Roy S, Barke RA, Loh HH (1998) MU-opioid receptor-knockout mice: role of mu-opioid receptor in morphine mediated immune functions. Brain Res Mol Brain Res 61(1–2):190–194. https://doi.org/10.1016/S0169-328X(98)00212-5
Roy S, Ninkovic J, Banerjee S, Charboneau RG, Das S, Dutta R, Kirchner VA, Koodie L et al (2011) Opioid drug abuse and modulation of immune function: consequences in the susceptibility to opportunistic infections. J NeuroImmune Pharmacol 6(4):442–465. https://doi.org/10.1007/s11481-011-9292-5
Chau DL, Walker V, Pai L, Cho LM (2008) Opiates and elderly: use and side effects. Clin Interv Aging 3(2):273–278. https://doi.org/10.2147/CIA.S1847
Roy S, Chapin RB, Cain KJ, Charboneau RG, Ramakrishnan S, Barke RA (1997) Morphine inhibits transcriptional activation of IL-2 in mouse thymocytes. Cell Immunol 179(1):1–9. https://doi.org/10.1006/cimm.1997.1147
Roy S, Cain KJ, Charboneau RG, Barke RA (1998) Morphine accelerates the progression of sepsis in an experimental sepsis model. Adv Exp Med Biol 437:21–31. https://doi.org/10.1007/978-1-4615-5347-2_3
Dinda A, Gitman M, Singhal PC (2005) Immunomodulatory effect of morphine: therapeutic implications. Expert Opin Drug Saf 4(4):669–675. https://doi.org/10.1517/14740338.4.4.669
Liu HC, Anday JK, House SD, Chang SL (2004) Dual effects of morphine on permeability and apoptosis of vascular endothelial cells: morphine potentiates lipopolysaccharide-induced permeability and apoptosis of vascular endothelial cells. J Neuroimmunol 146(1–2):13–21. https://doi.org/10.1016/j.jneuroim.2003.09.016
Wen H, Lu Y, Yao H, Buch S (2011) Morphine induces expression of platelet-derived growth factor in human brain microvascular endothelial cells: implication for vascular permeability. PLoS One 6(6):e21707. https://doi.org/10.1371/journal.pone.0021707
Hu S, Chao CC, Hegg CC, Thayer S, Peterson PK (2000) Morphine inhibits human microglial cell production of, and migration towards, RANTES. J Psychopharmacol 14(3):238–243. https://doi.org/10.1177/026988110001400307
Hu S, Sheng WS, Lokensgard JR, Peterson PK (2002) Morphine induces apoptosis of human microglia and neurons. Neuropharmacology 42(6):829–836. https://doi.org/10.1016/S0028-3908(02)00030-8
Horvath RJ, DeLeo JA (2009) Morphine enhances microglial migration through modulation of P2X4 receptor signaling. The Journal of Neuroscience: the Official Journal of the Society for Neuroscience 29(4):998–1005. https://doi.org/10.1523/JNEUROSCI.4595-08.2009
Hauser KF, Stiene-Martin A, Mattson MP, Elde RP, Ryan SE, Godleske CC (1996) Mu-opioid receptor-induced Ca2+ mobilization and astroglial development: morphine inhibits DNA synthesis and stimulates cellular hypertrophy through a Ca(2+)-dependent mechanism. Brain Res 720(1–2):191–203. https://doi.org/10.1016/0006-8993(96)00103-5
Bruce-Keller AJ, Turchan-Cholewo J, Smart EJ, Geurin T, Chauhan A, Reid R, Xu R, Nath A et al (2008) Morphine causes rapid increases in glial activation and neuronal injury in the striatum of inducible HIV-1 Tat transgenic mice. Glia 56(13):1414–1427. https://doi.org/10.1002/glia.20708
Tawfik VL, LaCroix-Fralish ML, Nutile-McMenemy N, DeLeo JA (2005) Transcriptional and translational regulation of glial activation by morphine in a rodent model of neuropathic pain. J Pharmacol Exp Ther 313(3):1239–1247. https://doi.org/10.1124/jpet.104.082420
Di Cesare Mannelli L, Corti F, Micheli L, Zanardelli M, Ghelardini C (2015) Delay of morphine tolerance by palmitoylethanolamide. Biomed Res Int 2015(894732):1–12. https://doi.org/10.1155/2015/894732
Childers SR, Snyder SH (1978) Guanine nucleotides differentiate agonist and antagonist interactions with opiate receptors. Life Sci 23(7):759–761. https://doi.org/10.1016/0024-3205(78)90077-2
Childers SR, Creese I, Snowman AM, Synder SH (1979) Opiate receptor binding affected differentially by opiates and opioid peptides. Eur J Pharmacol 55(1):11–18. https://doi.org/10.1016/0014-2999(79)90142-0
Diaz A, Ruiz F, Florez J, Pazos A, Hurle MA (1995) Regulation of dihydropyridine-sensitive Ca++ channels during opioid tolerance and supersensitivity in rats. J Pharmacol Exp Ther 274(3):1538–1544
Diaz A, Florez J, Pazos A, Hurle MA (2000) Opioid tolerance and supersensitivity induce regional changes in the autoradiographic density of dihydropyridine-sensitive calcium channels in the rat central nervous system. Pain 86(3):227–235. https://doi.org/10.1016/S0304-3959(00)00249-9
Nestler EJ (1996) Under siege: the brain on opiates. Neuron 16(5):897–900. https://doi.org/10.1016/S0896-6273(00)80110-5
Ippolito DL, Temkin PA, Rogalski SL, Chavkin C (2002) N-terminal tyrosine residues within the potassium channel Kir3 modulate GTPase activity of Galphai. J Biol Chem 277(36):32692–32696. https://doi.org/10.1074/jbc.M204407200
Torrecilla M, Quillinan N, Williams JT, Wickman K (2008) Pre- and postsynaptic regulation of locus coeruleus neurons after chronic morphine treatment: a study of GIRK-knockout mice. Eur J Neurosci 28(3):618–624. https://doi.org/10.1111/j.1460-9568.2008.06348.x
Wickman K, Clapham DE (1995) Ion channel regulation by G proteins. Physiol Rev 75(4):865–885. https://doi.org/10.1152/physrev.1995.75.4.865
Torrecilla M, Marker CL, Cintora SC, Stoffel M, Williams JT, Wickman K (2002) G-protein-gated potassium channels containing Kir3.2 and Kir3.3 subunits mediate the acute inhibitory effects of opioids on locus ceruleus neurons. J Neurosci 22(11):4328–4334
Volterra A, Meldolesi J (2005) Astrocytes, from brain glue to communication elements: the revolution continues. Nat Rev Neurosci 6(8):626–640. https://doi.org/10.1038/nrn1722
Abbott NJ, Ronnback L, Hansson E (2006) Astrocyte-endothelial interactions at the blood-brain barrier. Nat Rev Neurosci 7(1):41–53. https://doi.org/10.1038/nrn1824
Hadera MG, Eloqayli H, Jaradat S, Nehlig A, Sonnewald U (2015) Astrocyte-neuronal interactions in epileptogenesis. J Neurosci Res 93(7):1157–1164. https://doi.org/10.1002/jnr.23584
Wang DD, Bordey A (2008) The astrocyte odyssey. Prog Neurobiol 86(4):342–367. https://doi.org/10.1016/j.pneurobio.2008.09.015
Yan X, Shi ZF, LX X, Li JX, Wu M, Wang XX, Jia M, Dong LP et al (2017) Glutamate impairs mitochondria aerobic respiration capacity and enhances glycolysis in cultured rat astrocytes. Biomedical and environmental sciences : BES 30(1):44–51. https://doi.org/10.3967/bes2017.005
Hostenbach S, Cambron M, D'Haeseleer M, Kooijman R, De Keyser J (2014) Astrocyte loss and astrogliosis in neuroinflammatory disorders. Neurosci Lett 565:39–41. https://doi.org/10.1016/j.neulet.2013.10.012
Periyasamy P, Guo ML, Buch S (2016) Cocaine induces astrocytosis through ER stress-mediated activation of autophagy. Autophagy 12(8):1310–1329. https://doi.org/10.1080/15548627.2016.1183844
Guilarte TR, Nihei MK, McGlothan JL, Howard AS (2003) Methamphetamine-induced deficits of brain monoaminergic neuronal markers: distal axotomy or neuronal plasticity. Neuroscience 122(2):499–513. https://doi.org/10.1016/S0306-4522(03)00476-7
Narita M, Suzuki M, Kuzumaki N, Miyatake M, Suzuki T (2008) Implication of activated astrocytes in the development of drug dependence: differences between methamphetamine and morphine. Ann N Y Acad Sci 1141(1):96–104. https://doi.org/10.1196/annals.1441.032
Slezak M, Korostynski M, Gieryk A, Golda S, Dzbek J, Piechota M, Wlazlo E, Bilecki W et al (2013) Astrocytes are a neural target of morphine action via glucocorticoid receptor-dependent signaling. Glia 61(4):623–635. https://doi.org/10.1002/glia.22460
Lazriev IL, Kiknadze GI, Kutateladze II, Nebieridze MI (2001) Effect of morphine on the number and branching of astrocytes in various regions of rat brain. Bull Exp Biol Med 131(3):248–250. https://doi.org/10.1023/A:1017699315355
Ikeda H, Miyatake M, Koshikawa N, Ochiai K, Yamada K, Kiss A, Donlin MJ, Panneton WM et al (2010) Morphine modulation of thrombospondin levels in astrocytes and its implications for neurite outgrowth and synapse formation. J Biol Chem 285(49):38415–38427. https://doi.org/10.1074/jbc.M110.109827
Cai Y, Yang L, Hu G, Chen X, Niu F, Yuan L, Liu H, Xiong H et al (2016) Regulation of morphine-induced synaptic alterations: Role of oxidative stress, ER stress, and autophagy. J Cell Biol 215(2):245–258. https://doi.org/10.1083/jcb.201605065
Kim KH, Lee MS (2014) Autophagy--a key player in cellular and body metabolism. Nat Rev Endocrinol 10(6):322–337. https://doi.org/10.1038/nrendo.2014.35
Ryter SW, Cloonan SM, Choi AM (2013) Autophagy: a critical regulator of cellular metabolism and homeostasis. Molecules and cells 36(1):7–16. https://doi.org/10.1007/s10059-013-0140-8
Senft D, Ronai ZA (2015) UPR, autophagy, and mitochondria crosstalk underlies the ER stress response. Trends Biochem Sci 40(3):141–148. https://doi.org/10.1016/j.tibs.2015.01.002
Lindholm D, Wootz H, Korhonen L (2006) ER stress and neurodegenerative diseases. Cell Death Differ 13(3):385–392. https://doi.org/10.1038/sj.cdd.4401778
Cai Y, Arikkath J, Yang L, Guo ML, Periyasamy P, Buch S (2016) Interplay of endoplasmic reticulum stress and autophagy in neurodegenerative disorders. Autophagy 12(2):225–244. https://doi.org/10.1080/15548627.2015.1121360
Ogata M, Hino S, Saito A, Morikawa K, Kondo S, Kanemoto S, Murakami T, Taniguchi M et al (2006) Autophagy is activated for cell survival after endoplasmic reticulum stress. Mol Cell Biol 26(24):9220–9231. https://doi.org/10.1128/MCB.01453-06
Guo ML, Liao K, Periyasamy P, Yang L, Cai Y, Callen SE, Buch S (2015) Cocaine-mediated microglial activation involves the ER stress-autophagy axis. Autophagy 11(7):995–1009. https://doi.org/10.1080/15548627.2015.1052205
Roy S, Cain KJ, Chapin RB, Charboneau RG, Barke RA (1998) Morphine modulates NF kappa B activation in macrophages. Biochem Biophys Res Commun 245(2):392–396. https://doi.org/10.1006/bbrc.1998.8415
Zhao L, Zhu Y, Wang D, Chen M, Gao P, Xiao W, Rao G, Wang X et al (2010) Morphine induces Beclin 1- and ATG5-dependent autophagy in human neuroblastoma SH-SY5Y cells and in the rat hippocampus. Autophagy 6(3):386–394. https://doi.org/10.4161/auto.6.3.11289
El-Hage N, Rodriguez M, Dever SM, Masvekar RR, Gewirtz DA, Shacka JJ (2015) HIV-1 and morphine regulation of autophagy in microglia: limited interactions in the context of HIV-1 infection and opioid abuse. J Virol 89(2):1024–1035. https://doi.org/10.1128/JVI.02022-14
Kimura S, Noda T, Yoshimori T (2007) Dissection of the autophagosome maturation process by a novel reporter protein, tandem fluorescent-tagged LC3. Autophagy 3(5):452–460. https://doi.org/10.4161/auto.4451
Schneider CA, Rasband WS, Eliceiri KW (2012) NIH Image to ImageJ: 25 years of image analysis. Nat Methods 9(7):671–675. https://doi.org/10.1038/nmeth.2089
Pare EM, Monforte JR, Thibert RJ (1984) Morphine concentrations in brain tissue from heroin-associated deaths. J Anal Toxicol 8(5):213–216. https://doi.org/10.1093/jat/8.5.213
Yu B, Wenjun Z, Changsheng Y, Yuntao F, Jing M, Ben L, Hai Q, Guangwei X et al (2016) Preconditioning of endoplasmic reticulum stress protects against acrylonitrile-induced cytotoxicity in primary rat astrocytes: the role of autophagy. Neurotoxicology 55:112–121. https://doi.org/10.1016/j.neuro.2016.05.020
Cao L, Walker MP, Vaidya NK, Fu M, Kumar S, Kumar A (2016) Cocaine-mediated autophagy in astrocytes involves sigma 1 receptor, PI3K, mTOR, Atg5/7, Beclin-1 and induces type II programed cell death. Mol Neurobiol 53(7):4417–4430. https://doi.org/10.1007/s12035-015-9377-x
Kubota K, Niinuma Y, Kaneko M, Okuma Y, Sugai M, Omura T, Uesugi M, Uehara T et al (2006) Suppressive effects of 4-phenylbutyrate on the aggregation of Pael receptors and endoplasmic reticulum stress. J Neurochem 97(5):1259–1268. https://doi.org/10.1111/j.1471-4159.2006.03782.x
Rozpedek W, Pytel D, Mucha B, Leszczynska H, Diehl JA, Majsterek I (2016) The role of the PERK/eIF2alpha/ATF4/CHOP signaling pathway in tumor progression during endoplasmic reticulum stress. Curr Mol Med 16(6):533–544. https://doi.org/10.2174/1566524016666160523143937
Fukagawa H, Koyama T, Kakuyama M, Fukuda K (2013) Microglial activation involved in morphine tolerance is not mediated by toll-like receptor 4. J Anesth 27(1):93–97. https://doi.org/10.1007/s00540-012-1469-4
Tauber SC, Staszewski O, Prinz M, Weis J, Nolte K, Bunkowski S, Bruck W, Nau R (2016) HIV encephalopathy: glial activation and hippocampal neuronal apoptosis, but limited neural repair. HIV Med 17(2):143–151. https://doi.org/10.1111/hiv.12288
Wang X, Loram LC, Ramos K, de Jesus AJ, Thomas J, Cheng K, Reddy A, Somogyi AA et al (2012) Morphine activates neuroinflammation in a manner parallel to endotoxin. Proc Natl Acad Sci U S A 109(16):6325–6330. https://doi.org/10.1073/pnas.1200130109
Wan J, Ma J, Anand V, Ramakrishnan S, Roy S (2015) Morphine potentiates LPS-induced autophagy initiation but inhibits autophagosomal maturation through distinct TLR4-dependent and independent pathways. Acta Physiol 214(2):189–199. https://doi.org/10.1111/apha.12506
Pan Y, Sun X, Jiang L, Hu L, Kong H, Han Y, Qian C, Song C et al (2016) Metformin reduces morphine tolerance by inhibiting microglial-mediated neuroinflammation. J Neuroinflammation 13(1):294. https://doi.org/10.1186/s12974-016-0754-9
Dalvi P, Sharma H, Chinnappan M, Sanderson M, Allen J, Zeng R, Choi A, O’Brien-Ladner A et al (2016) Enhanced autophagy in pulmonary endothelial cells on exposure to HIV-Tat and morphine: role in HIV-related pulmonary arterial hypertension. Autophagy 12(12):2420–2438. https://doi.org/10.1080/15548627.2016.1238551
Dever SM, Rodriguez M, Lapierre J, Costin BN, El-Hage N (2015) Differing roles of autophagy in HIV-associated neurocognitive impairment and encephalitis with implications for morphine co-exposure. Front Microbiol 6:653. https://doi.org/10.3389/fmicb.2015.00653
Saitoh T, Fujita N, Jang MH, Uematsu S, Yang BG, Satoh T, Omori H, Noda T et al (2008) Loss of the autophagy protein Atg16L1 enhances endotoxin-induced IL-1beta production. Nature 456(7219):264–268. https://doi.org/10.1038/nature07383
Paul S, Kashyap AK, Jia W, He YW, Schaefer BC (2012) Selective autophagy of the adaptor protein Bcl10 modulates T cell receptor activation of NF-kappaB. Immunity 36(6):947–958. https://doi.org/10.1016/j.immuni.2012.04.008
Henckaerts L, Cleynen I, Brinar M, John JM, Van Steen K, Rutgeerts P, Vermeire S (2011) Genetic variation in the autophagy gene ULK1 and risk of Crohn’s disease. Inflamm Bowel Dis 17(6):1392–1397. https://doi.org/10.1002/ibd.21486
Nakamura S, Yoshimori T (2017) New insights into autophagosome-lysosome fusion. J Cell Sci 130(7):1209–1216. https://doi.org/10.1242/jcs.196352
Button RW, Roberts SL, Willis TL, Hanemann CO, Luo S (2017) Accumulation of autophagosomes confers cytotoxicity. J Biol Chem 292(33):13599–13614. https://doi.org/10.1074/jbc.M117.782276
Hori I, Otomo T, Nakashima M, Miya F, Negishi Y, Shiraishi H, Nonoda Y, Magara S et al (2017) Defects in autophagosome-lysosome fusion underlie Vici syndrome, a neurodevelopmental disorder with multisystem involvement. Sci Rep 7(1):3552. https://doi.org/10.1038/s41598-017-02840-8
Schroder M (2008) Endoplasmic reticulum stress responses. Cell Mol Life Sci 65(6):862–894. https://doi.org/10.1007/s00018-007-7383-5
Kroemer G, Marino G, Levine B (2010) Autophagy and the integrated stress response. Mol Cell 40(2):280–293. https://doi.org/10.1016/j.molcel.2010.09.023
B'Chir W, Maurin AC, Carraro V, Averous J, Jousse C, Muranishi Y, Parry L, Stepien G et al (2013) The eIF2alpha/ATF4 pathway is essential for stress-induced autophagy gene expression. Nucleic Acids Res 41(16):7683–7699. https://doi.org/10.1093/nar/gkt563
Kouroku Y, Fujita E, Tanida I, Ueno T, Isoai A, Kumagai H, Ogawa S, Kaufman RJ et al (2007) ER stress (PERK/eIF2alpha phosphorylation) mediates the polyglutamine-induced LC3 conversion, an essential step for autophagy formation. Cell Death Differ 14(2):230–239. https://doi.org/10.1038/sj.cdd.4401984
Hoyer-Hansen M, Bastholm L, Szyniarowski P, Campanella M, Szabadkai G, Farkas T, Bianchi K, Fehrenbacher N et al (2007) Control of macroautophagy by calcium, calmodulin-dependent kinase kinase-beta, and Bcl-2. Mol Cell 25(2):193–205. https://doi.org/10.1016/j.molcel.2006.12.009
Yousefi S, Perozzo R, Schmid I, Ziemiecki A, Schaffner T, Scapozza L, Brunner T, Simon HU (2006) Calpain-mediated cleavage of Atg5 switches autophagy to apoptosis. Nat Cell Biol 8(10):1124–1132. https://doi.org/10.1038/ncb1482
Liu S, Sarkar C, Dinizo M, Faden AI, Koh EY, Lipinski MM, Wu J (2015) Disrupted autophagy after spinal cord injury is associated with ER stress and neuronal cell death. Cell Death Dis 6(1):e1582. https://doi.org/10.1038/cddis.2014.527
Ye J, Jiang Z, Chen X, Liu M, Li J, Liu N (2017) The role of autophagy in pro-inflammatory responses of microglia activation via mitochondrial reactive oxygen species in vitro. J Neurochem 142(2):215–230. https://doi.org/10.1111/jnc.14042
Aoe T (2013) Endoplasmic reticulum stress and opioid tolerance withdrawal. Masui the Japanese Journal of Anesthesiology 62(3):283–289
Dobashi T, Tanabe S, Jin H, Nishino T, Aoe T (2010) Valproate attenuates the development of morphine antinociceptive tolerance. Neurosci Lett 485(2):125–128. https://doi.org/10.1016/j.neulet.2010.08.084
Ghavimi H, Hassanzadeh K, Maleki-Dizaji N, Azarfardian A, Ghasami S, Zolali E, Charkhpour M (2014) Pioglitazone prevents morphine antinociception tolerance and withdrawal symptoms in rats. Naunyn Schmiedeberg's Arch Pharmacol 387(9):811–821. https://doi.org/10.1007/s00210-014-0996-y
Campbell LA, Avdoshina V, Rozzi S, Mocchetti I (2013) CCL5 and cytokine expression in the rat brain: differential modulation by chronic morphine and morphine withdrawal. Brain Behav Immun 34:130–140. https://doi.org/10.1016/j.bbi.2013.08.006
Amaral GF, Dossa PD, Viebig LB, Konno FTC, Consoli A, Martins MFM, Viani FC, Bondan EF (2016) Astrocytic expression of GFAP and serum levels of IL-12 and TNF-± in rats treated with different pain relievers. Brazilian Journal of Pharmaceutical Sciences 52(4):623–633. https://doi.org/10.1590/s1984-82502016000400006
Beitner-Johnson D, Guitart X, Nestler EJ (1993) Glial fibrillary acidic protein and the mesolimbic dopamine system: regulation by chronic morphine and Lewis-Fischer strain differences in the rat ventral tegmental area. J Neurochem 61(5):1766–1773. https://doi.org/10.1111/j.1471-4159.1993.tb09814.x
Patel NA, Romero AA, Zadina JE, Chang SL (1996) Chronic exposure to morphine attenuates expression of interleukin-1 beta in the rat hippocampus. Brain Res 712(2):340–344. https://doi.org/10.1016/0006-8993(95)01575-2
Acknowledgements
We are grateful to Drs. Guoku Hu, Ernest Chivero, Annadurai Thangaraj, and Mr. Ke Liao for their useful discussions and to Ms. Fang Niu and Ms. Yeon Hee Kook for their technical assistance.
Funding
This work was supported by grants DA033614, DA035203, and DA041751 (SB) from the National Institutes of Health.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no competing interests.
Electronic Supplementary Material
Supplementary Fig. 1
(PDF 60 kb)
Rights and permissions
About this article
Cite this article
Sil, S., Periyasamy, P., Guo, ML. et al. Morphine-Mediated Brain Region-Specific Astrocytosis Involves the ER Stress-Autophagy Axis. Mol Neurobiol 55, 6713–6733 (2018). https://doi.org/10.1007/s12035-018-0878-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-018-0878-2