Abstract
Neurotrophic factors (NTFs) hold potential as disease-modifying therapies for neurodegenerative disorders like Parkinson’s disease. Glial cell line-derived neurotrophic factor (GDNF), cerebral dopamine neurotrophic factor (CDNF), and mesencephalic astrocyte-derived neurotrophic factor (MANF) have shown neuroprotective and restorative effects on nigral dopaminergic neurons in various animal models of Parkinson’s disease. To date, however, their effects on brain neurochemistry have not been compared using in vivo microdialysis. We measured extracellular concentration of dopamine and activity of dopamine neurochemistry-regulating enzymes in the nigrostriatal system of rat brain. NTFs were unilaterally injected into the striatum of intact Wistar rats. Brain microdialysis experiments were performed 1 and 3 weeks later in freely-moving animals. One week after the treatment, we observed enhanced stimulus-evoked release of dopamine in the striatum of MANF-treated rats, but not in rats treated with GDNF or CDNF. MANF also increased dopamine turnover. Although GDNF did not affect the extracellular level of dopamine, we found significantly elevated tyrosine hydroxylase (TH) and catechol-O-methyltransferase (COMT) activity and decreased monoamine oxidase A (MAO-A) activity in striatal tissue samples 1 week after GDNF injection. The results show that GDNF, CDNF, and MANF have divergent effects on dopaminergic neurotransmission, as well as on dopamine synthetizing and metabolizing enzymes. Although the cellular mechanisms remain to be clarified, knowing the biological effects of exogenously administrated NTFs in intact brain is an important step towards developing novel neurotrophic treatments for degenerative brain diseases.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Parkinson’s disease (PD) is a progressive neurodegenerative disorder which holds an unmet need for a curative treatment. It is the most common degenerative brain disease after Alzheimer’s disease affecting approximately 2–3% of the population over 65 years of age [1]. The essential neuropathological hallmarks of PD are death of dopaminergic cell bodies in the midbrain substantia nigra pars compacta and presence of intraneuronal cytoplasmic aggregates containing misfolded α-synuclein called Lewy bodies and Lewy neurites [2, 3]. The characteristic parkinsonian motor symptoms, including bradykinesia, rigidity, resting tremor, and postural impairment, result from the degeneration of the nigrostriatal dopaminergic neurons and resultant dopamine deficiency within the dorsal striatum.
Neurotrophic factors (NTFs) are endogenous secretory proteins which promote differentiation, maintenance, function, and plasticity of the nervous system and help neurons to recover after an injury [4,5,6,7]. Due to these trophic effects, NTFs are considered as potential disease-modifying therapies for neurodegenerative disorders such as PD. Glial cell line-derived neurotrophic factor (GDNF), cerebral dopamine neurotrophic factor (CDNF), and mesencephalic astrocyte-derived neurotrophic factor (MANF) have shown neuroprotective and neurorestorative effects on lesioned dopaminergic neurons in vitro and in various animal models of PD [8,9,10,11,12,13,14,15,16,17,18]. In in vivo lesion models, these NTFs increase the survival of midbrain dopamine cells and fibers and improve aberrant motor performance suggesting enhanced dopaminergic function. However, if we want to look upon NTFs as a novel therapeutic approach for PD, it is crucial to understand how exogenously administered, non-physiological concentrations of NTFs influence the normal nigrostriatal neurochemistry and neurotransmitter homeostasis.
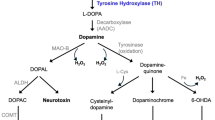
GDNF is a distant member of the TGF-β superfamily of growth factors and conveys its biological effects via receptor tyrosine kinase RET as Airaksinen and Saarma have comprehensively reviewed [5]. GDNF has been shown to potentiate the excitability of dopaminergic neurons and increase the release of dopamine in cell cultures and midbrain slices [19,20,21,22]. Intracranial administration of GDNF elevated stimulus-evoked dopamine overflow in the striatum of rats and rhesus monkeys [23,24,25,26,27,28,29]. However, the major limitation in the earlier in vivo studies is that they have been conducted under general anesthesia which is known to have marked effects on neurotransmission, inducing alterations in neuronal activity, neurotransmitter synthesis, release, reuptake, and metabolism [30, 31]. To the best of our knowledge, the ability of GDNF to alter dopamine release in completely non-anesthetized animals has not been studied before.
CDNF and MANF form an evolutionary conserved and structurally distinct family of NTFs [6, 12, 13]. CDNF and MANF are located intracellularly in the endoplasmic reticulum (ER), but they can be also secreted from cells [32,33,34]. Their mechanism of action is still unclear, although growing body of evidence suggests that CDNF and MANF play an important role in the maintenance of protein homeostasis in the ER and alleviate/regulate ER stress [35,36,37,38,39,40,41]. Thus far, however, the effects of intracerebrally administrated CDNF or MANF on dopaminergic neurotransmission in vivo have remained unstudied.
It has been shown that an intrastriatal injection of GDNF as well as long-term overexpression of GDNF downregulate tyrosine hydroxylase (TH), the rate-limiting enzyme in dopamine biosynthesis, in the midbrain of adult rats without affecting the total activity of the enzyme [42,43,44]. On the other hand, GDNF administration has been reported to increase phosphorylation of TH at Ser31 and Ser40—the serine residues controlling TH activity [28, 42, 45, 46]. To date, nothing is known about the effects of CDNF or MANF on TH activity, nor the effect of GDNF on dopamine metabolizing enzymes catechol-O-methyltransferase (COMT) and monoamine oxidases A and B (MAO-A and MAO-B).
The aim of the present study was to elucidate the effects of intrastriatally administrated GDNF, CDNF, and MANF proteins on dopamine release within the dorsal striatum of intact freely-moving rats. We also wanted to clarify the effect of a single intrastriatal injection of GDNF, CDNF, and MANF on in vivo activity of TH. Finally, based on the results of the microdialysis experiments, we tested the hypothesis that GDNF alters the activity of COMT, MAO-A, and MAO-B. Our results revealed divergent changes in dopamine release as well as in dopamine synthesis and metabolism after exogenously administrated NTFs. This is highly relevant information when regarding NTFs as novel therapeutic approaches for PD.
Materials and Methods
Experimental Animals
Male Wistar rats (RccHan:WIST, Harlan, the Netherlands), weighing 230–490 g during stereotaxic surgery, were used for all experiments. For microdialysis experiments, rats were moved from group housing (3–4 animals) to individual test cages (30 × 30 × 30 cm) after the surgery. Tap water and rat chow (Tekland Global Diet, Harlan) were available ad libitum. Rats were under a 12:12 h light-dark cycle (lights on at 6:20 am), at an ambient temperature of 20–22 °C. Stereotaxic surgeries and experiments were performed during daylight between 8:00 am and 6:00 pm. All animal experiments were in accordance with the directive of the European Parliament and of the Council on the protection of animals used for scientific purposes (Directive 2010/63/EU of the European Parliament and of the Council). Experiments were approved by the National Animal Experiment Board of Finland (ESLH-2009-05234/Ym-23 and ESAVI/198/04.10.07/2014).
Neurotrophic Factors
Vehicle group received sterile phosphate-buffered saline (PBS). Recombinant hGDNF (2 μg/μl, reconstituted with Milli-Q® water according to the manufacturer’s instructions) was produced in E. coli bacterial cells (ProSpec-Tany TechnoGene Ltd., Israel). The purity of GDNF was greater than 95% as determined by SDS-PAGE electrophoresis and RP-HPLC. GDNF was tested by the manufacturer to be compliant for cell culture use in terms of lipopolysaccharide (LPS) counts in the protein sample. Recombinant hCDNF (2 μg/μl, in PBS) was produced in Spodoptera frugiperda (Sf9) insect cells (Biovian Oy, Finland) and recombinant hMANF (2 μg/μl, in PBS) in Chinese Hamster Ovary (CHO) mammalian cells (Icosagen AS, Estonia). Both CDNF and MANF were purified from serum-free cell supernatant using ion-exchange chromatography. The purity of CDNF and MANF was greater than 95% as determined by SDS-PAGE electrophoresis and mass-spectrometry.
Stereotaxic Surgery
Stereotaxic surgeries were performed under isoflurane (Attane Vet 1000 mg/g, Piramal Healthcare, UK) anesthesia (3.5–4.5% during induction and 2.0–3.5% during maintenance). Rats were fixed on a stereotaxic frame (Stoelting Co., IL, USA), and the skull was exposed. Lidocaine-adrenalin solution (10 mg/ml, Orion Pharma Oyj, Finland) was used for local anesthesia and to prevent bleeding. A burr hole was made using a high-speed dental drill (Foredom SR, The Foredom Electric Co., CT, USA). A unilateral injection of GDNF, CDNF, or MANF (10 μg in 5 μl) or PBS as vehicle (5 μl) was made into the left dorsal striatum (A/P + 1.0; M/L + 2.7; D/V − 5.0 relative to the bregma, according to the rat brain atlas [47]) using an electronic microinjector (Quintessential stereotactic injector, Stoelting) and a 10-μl microsyringe (Hamilton Company, NV, USA) with a 26 gauge blunt tapered needle. The injection rate was set to 1 μl/min. At the completion of the injection, the needle was kept in place for 4 min to minimize backflow of the solution. For the microdialysis experiments, a guide cannula (BASi MD-2250, Bioanalytical Systems Inc., IN, USA) was implanted right after the NTF or vehicle injection. The tip of the cannula was lowered into the left dorsal striatum (A/P + 1.0; M/L + 2.7; D/V − 4.0 relative to the bregma, according to the rat brain atlas [47]) after which the cannula was attached to the skull with three stainless steel screws and dental cement (Aqualox, Voco Cuxhaven GmbH, Germany). To relieve postoperative pain, rats received tramadol 1 mg/kg s.c. (Tramal 50 mg/ml, Orion Pharma) at the end of the surgery, and another similar injection 12 h later if needed. After the surgery, rats were allowed to recover for 7 days before the first microdialysis experiment.
Microdialysis
Microdialysis experiments were carried out in freely-moving rats 1 and 3 weeks after the stereotaxic surgery. Before experiments, all probes (BASi MD-2200, Bioanalytical Systems, membrane length 2 mm) were tested for in vitro recovery at room temperature to ensure their proper function. However, in vivo dialysate concentrations were not corrected for in vitro recoveries because corrected concentrations do not correlate to true analyte concentrations in extracellular fluid [48]. In vitro recoveries of the probes for dopamine ranged from 7.3 to 19.1%. Before the experiments, there was a 2-h stabilization period: the probe was inserted into the guide cannula, and perfusion of the membrane was started with modified Ringer solution (147 mM NaCl, 2.7 mM KCl, 1.2 mM CaCl2, 1.0 mM MgCl2, 0.04 mM ascorbic acid) at a flow rate of 2 μl/min. After the stabilization period, dialysate samples were collected every 15 min for 270 min (Fig. 1b). The samples were analyzed right after collecting using a high-performance liquid chromatography (HPLC) coupled with an electrochemical detector as described below. Analyte concentrations in the first four samples (time points 15, 30, 45, and 60 min) were used to calculate baseline levels (as averages) for dopamine and its main metabolites 3,4-dihydroxyphenylacetic acid (DOPAC) and homovanillic acid (HVA). To stimulate dopamine release from nerve terminals, two different stimulation solutions were administrated via reverse dialysis. First, the Ringer solution was changed into 100 mM potassium solution (27.5 mM NaCl, 100 mM KCl, 1.2 mM CaCl2, 1.0 mM MgCl2, 0.04 mM ascorbic acid) at time point 15 min. This high-potassium solution was pumped for 15 min after which it was changed back to the Ringer solution. At time point 120 min, the Ringer solution was replaced with 100 μM D-amphetamine solution for 15 min. After this, the Ringer solution was used until the end of the experiment. The results were analyzed as percent changes of the analyte concentrations compared to the corresponding baseline levels. If the concentration of any analyte in the last two baseline samples (time points 45 and 60 min) differed more than 20% of the average, the rat was excluded from the data. After the first microdialysis experiment, the probe was removed from the brain. After the second experiment, rat was sacrificed and the brain was excised and frozen on dry ice. The correct placements of the microdialysis probes were verified histologically from 90-μm-thick coronal brain sections which were cut with a sliding microtome (Leica CM3050, Leica Instruments GmbH, Germany) and fixed on gelatin-chrome-alume-coated microscope slides. Data only from the rats with accurate probe placements in the dorsal striatum were included in the analyses.
Design of the study and time course of the dopamine microdialysis experiments. a All rats received an intrastriatal, unilateral injection of GDNF, CDNF, MANF, or vehicle. One cohort of rats underwent dopamine microdialysis experiments 1 and 3 weeks after the NTF injection. Another cohort of rats was used to collect striatal tissue samples for in vivo TH activity measurements 1 week after the NTF treatment. NSD1015 (3-hydroxybenzylhydrazine) was administrated i.p. to inhibit aromatic amino acid dopa decarboxylase in the brain 30 min before tissue collection. From the third cohort of rats, striatal tissue samples were collected for ex vivo COMT and MAO activity measurements 1 week after the NTF treatment. b In the microdialysis experiments, samples were collected every 15 min for 270 min. The first four samples collected at 15, 30, 45, and 60 min were used to calculate baseline levels. Then, dopamine release was stimulated via reverse dialysis of two different stimulation solutions. First high-potassium (100 mM) perfusion solution was pumped for 15 min at time point 15 min (K+ administration) which led to K+ response between time points 75 and 135 min. At time point 120 min, amphetamine (100 μM)-containing perfusion solution was pumped for 15 min (Amphet. administration) which led to amphetamine response between time points 180 and 255 min. The lag time between the administration of a stimulation solution and the corresponding response is due to slow flow rate of the perfusion solution (2 μl/min) through microdialysis tubes which have to be long in case of freely-moving animals (i.e., dead volume of the tubes is high)
Quantification of Dopamine and Metabolites from Microdialysis Samples
The analysis of samples collected in microdialysis experiments and in in vitro recovery tests was performed with slight modifications from the methods described earlier [49, 50]. The concentrations of dopamine, DOPAC and HVA in the samples were analyzed with a HPLC system equipped with an ESA Coulochem II electrochemical detector and a model 5014B microdialysis cell (ESA Biosciences Inc., MA, USA). Dopamine was reduced with an amperometric detector (potential − 100 mV) after being oxidized with a coulometric detector (+ 300 mV); DOPAC and HVA were oxidized with the coulometric detector. Dialysate samples of 25 μl were injected into the column (Kinetex C18, 2.6 μm, 100 Å, 50 × 4.6 mm; Phenomenex Inc., CA, USA) with an autoinjector (Prominence, SIL-20 AC, Shimadzu Co., Japan). The column was protected with SecurityGuard Ultra filter (Phenomenex) and its temperature was kept at 45 °C with a column heater (Croco-Cil, Cluzeau Info Labo, France). The mobile phase was a mixture of 0.1 M NaH2PO4 buffer, pH 4.0, 100 mg/l octanesulphonic acid, approximately 5% (v/v) of methanol and 0.2 M ethylenediaminetetraacetic acid (EDTA). The flow rate of the mobile phase was kept constant at 1.0 ml/min with an isocratic pump (Jasco PU-2080 Plus, Jasco Co., Essex, UK) equipped with two pulse dampers (SSI LP-21, Scientific Systems Inc., PA, USA). The chromatograms were processed with chromatogram integration software (Azur 4.0, Datalys, France).
In Vivo TH Activity Experiment
Seven days after an intrastriatal injection of NTFs (10 μg in 5 μl) or vehicle (5 μl), rats were administered with 3-hydroxybenzylhydrazine (NSD1015) 100 mg/kg, i.p. (Sigma-Aldrich Chemie GmbH, Germany) to inhibit aromatic amino acid dopa decarboxylase (AADC) in the brain [51]. Rats were decapitated 30 min after the NSD1015 injection; the brains were excised rapidly and rinsed with ice-cold physiological saline solution. Dorsal striatum samples were collected bilaterally from 2-mm coronal slices using an ice-cooled rat brain matrix (Stoelting) and a 3-mm sample corer (Fine Science Tools GmbH, Heildelberg, Germany) and frozen immediately on dry ice. The samples were weighed and homogenized with a sonicator (GM35-400, Rinco Ultrasonics AG, Switzerland) in 500 μl of homogenization solution consisting of six parts of 0.2 M HClO4 and one part of antioxidant solution containing 1.0 mM oxalic acid, 0.1 M acetic acid, and 3.0 mM L-cysteine [52]. The homogenates were centrifuged at 20,800×g for 35 min at 4 °C (Eppendorf Centrifuge 5810R, Eppendorf AG, Germany). After the centrifuging, 300 μl of the supernatant was moved into Vivaspin® 500 filter concentrators (10,000 MWCO PES; Sartorius Stedim Biotech GmbH, Germany) and centrifuged again at 8600×g for 35 min at 4 °C. Filtrates containing monoamines were analyzed with a HPLC system as described below. The amount of L-3,4-dihydroxyphenylalanine (L-DOPA) in the striatum samples was calculated as nanograms per gram (ng/g) wet weight of the sample for both hemispheres.
Quantification of L-DOPA from Striatal Tissue Samples
The concentration of L-DOPA in striatal tissue samples was measured with a HPLC system as described earlier [53]. Samples of 100 μl were injected into the column (Kinetex XD-C18, 2.6 μm, 100 Å, 100 × 4.6 mm; Phenomenex) with an autoinjector (Prominence Auto Sampler, SIL-20 AC, Shimadzu). The column temperature was kept at 45 °C with a column heater (Croco-Cil). The mobile phase was a mixture of 0.1 M NaH2PO4 buffer, pH 3.0, 150 mg/l octanesulfonic acid 4% (v/v) of methanol. An isocratic pump (ESA Model 582 Solvent Delivery Module; ESA Biosciences) equipped with a pulse damper (SSI LP-21, Scientific Systems) provided a constant flow rate of 1.0 ml/min. The analytes were detected with an electrochemical detector (ESA CoulArray Electrode Array, ESA Biosciences), and the chromatograms were processed with an integration software (CoulArray for Windows, ESA Biosciences).
Estimation of COMT and MAO Activities
COMT, MAO-A, and MAO-B enzyme activities were measured from striatum samples 7 days after an intrastriatal injection of GDNF (10 μg in 5 μl) or vehicle (5 μl). Rats were decapitated, and the brains were excised rapidly and rinsed with ice-cold physiological saline solution. Dorsal striatum samples were collected bilaterally from 4-mm coronal slices using an ice-cooled rat brain matrix (Stoelting) and a 3-mm sample corer (Fine Science Tools) and frozen immediately on dry ice. The samples were weighed and homogenized with a sonicator (GM35-400, Rinco Ultrasonics) in 10 mM phosphate buffer (pH 7.4) in a volume of 20× wet weight of the sample. The homogenates were centrifuged at 1000×g for 20 min at 4 °C (Eppendorf Centrifuge 5810R). Total protein concentration was determined using bicinchoninic acid method (Pierce™ BCA Protein Assay Kit, Thermo Fisher Scientific Inc., MA, USA).
Total COMT activity assay was performed as described earlier [54, 55]. The enzyme suspension was incubated for 30 min at 37 °C in 100 mM phosphate buffer (pH 7.4) containing 5 mM MgCl2, 200 μM S-adenosyl-L-methionine (Sigma Chemical Co., MO, USA), and 500 μM 3,4-dihydroxybenzoic acid (Sigma). The reaction was stopped by adding ice-cold 4 M perchloric acid. Samples were centrifuged at 5530×g for 10 min at 4 °C after which supernatant was filtered through 0.45-μm syringe filter (Millex®-HA, Millipore, Merck KGaA, Darmstadt, Germany) and diluted 1:20 in 0.4 M perchloric acid. The reaction products, vanillic and isovanillic acid, were analyzed with a HPLC system equipped with an ESA Coulochem II electrochemical detector (detector potential + 500 mV) and a model 5011A analytical cell (ESA Biosciences). An autoinjector (Prominence SIL-20AC, Shimadzu) was used to inject 10 μl of the samples into the column (Spherisorb® ODS2, C18, 3 μm, 4.6 × 100 mm; Waters Spherisorb, MA, USA). The column temperature was kept at 35 °C with a column heater (Croco-Cil). The mobile phase consisted of 0.1 M Na2HPO4 buffer (pH 3.3), 0.15 mM EDTA and 25% methanol. The flow rate of the mobile phase was set to 1.0 ml/min and kept constant with an isocratic pump (Jasco PU-2080 Plus, Jasco). The chromatograms were processed with an integration software (Azur 5.0, Datalys). Total COMT activity was calculated as picomoles of vanillic acid formed in 1 minute per milligram of protein in the sample.
MAO-A and MAO-B activities were determined with Monoamine Oxidase Assay Kit (MAK136, Sigma-Aldrich) according to the manufacturer’s instructions. In the assay, MAO-A and -B react with p-tyramine forming H2O2 which is determined by a fluorimetric method. To isolate MAO-A and MAO-B activities, isoform-specific inhibitors were used: MAO-A activity was assayed by adding 5 μM of MAO-A-specific inhibitor clorgyline into the control well and subtracting the remaining MAO-B activity from the total MAO activity in the sample well without inhibitors. Likewise, MAO-B activity was assayed by adding 5 μM of MAO-B-specific inhibitor pargyline into the control well and subtracting the remaining MAO-A activity from the total MAO activity. Black 96-well plates with clear bottom were used in the assay. The fluorescence of the samples and H2O2 standard curve was measured with a multi-well plate reader (Victor2, 1420 Multilabel Plate Reader, PerkinElmer Inc., MA, USA) using an excitation wavelength of 530 nm and a detection wavelength of 590 nm.
Statistical Analysis
Data from the microdialysis experiments were analyzed with analysis of variance (ANOVA) for repeated measures followed by Ryan-Einot-Gabriel-Welsch F (REGWF) post hoc test. One-way ANOVA followed by REGWF post hoc test was used to compare the differences in the baseline concentrations between the treatment groups as well as to analyze results from the in vivo TH activity experiment. Differences in the baseline concentrations within the treatment groups 1 and 3 weeks after the surgery were analyzed with paired two-tailed Student’s t test. Results from the COMT and MAO activity experiments were analyzed with unpaired two-tailed Student’s t tests. All analyses were conducted with SPSS® Statistics 24 software (IBM SPSS Inc., IL, USA). Exclusion criterion used in the data analyses was mean ± 2 × standard deviation. Results are expressed as mean ± SEM and considered to be significant at p < 0.05.
Results
Unaltered Baseline Levels of Dopamine and Its Metabolites After NTF Treatments
The effects of intrastriatally injected NTFs on dopamine release from nigrostriatal dopaminergic neurons were examined in two consecutive microdialysis experiments, at 1 and 3 weeks after the stereotaxic surgery, in freely-moving rats. The extracellular baseline levels of dopamine, DOPAC, or HVA did not differ significantly between the treatment groups either at 1 or 3 weeks after the surgery. However, the baseline levels of dopamine and its metabolites were significantly lower in most of the treatment groups when measured at 3 weeks after the surgery as compared with the concentrations measured at 1 week after the surgery (Table 1).
Elevated Stimulus-Evoked Release of Dopamine in MANF-Treated Rats
To study the ability of NTFs to alter stimulus-evoked release of dopamine in the striatum, dopaminergic nerve terminals were first depolarized by administrating high concentration of potassium via reverse dialysis which caused an extensive increase in the extracellular concentration of dopamine in all treatment groups (Fig. 2a, b). After a recovery period, administration of amphetamine through the microdialysis probe drained dopaminergic vesicles and reversed the function of dopamine transporter (DAT) in the nerve terminals [56], thus inducing another notable increase in dopamine release.
Extracellular striatal concentrations of dopamine and its main metabolites 3,4-dihydroxyphenylacetic acid (DOPAC) and homovanillic acid (HVA) measured using brain microdialysis 1 week after an intrastriatal injection of GDNF, CDNF, MANF, or vehicle. a In MANF-treated rats, both potassium-evoked (75–135 min) and amphetamine-evoked (180–255 min) release of dopamine was elevated when compared to vehicle- and GDNF-treated rats. In addition, the overall (15–270 min) extracellular dopamine concentration was elevated in MANF group compared to vehicle and GDNF groups, and in CDNF group compared to GDNF group (*p < 0.05 MANF vs. VEH and MANF vs. GDNF; #p < 0.05 CDNF vs. GDNF; REGWF post hoc analysis after ANOVA for repeated measures). b Average dopamine overflow during potassium and amphetamine responses was augmented in MANF-treated rats when compared to vehicle- and GDNF-treated rats (*p < 0.05 MANF vs. VEH and MANF vs. GDNF; REGWF post hoc analysis after one-way ANOVA). c, d NTFs did not have significant effects on extracellular DOPAC or HVA concentration at 1 week after the injection. The period when high-potassium and amphetamine perfusion solutions were pumped (K+ and Amphet. administration) (a) and the period of potassium and amphetamine responses (a, c, d) are depicted under the x-axes. Results are shown as % of baseline value (= 100%); mean ± SEM; n = 10 in each group
One week after the surgery, potassium- and amphetamine-evoked release of dopamine was significantly elevated in MANF-treated rats as compared with the vehicle- and GDNF-treated animals (ANOVA for repeated measures 75–135 min: F3,36 = 4.874; p = 0.006; REGWF p < 0.05; 180–255 min: F3,36 = 3.683; p = 0.021; REGWF p < 0.05) (Fig. 2a). In addition, extracellular dopamine concentration significantly differed between the treatment groups during the whole experiment (ANOVA for repeated measures 15–270 min: F3,36 = 4.678; p = 0.007). According to REGWF’s post hoc test, the differences were between MANF- and vehicle-, MANF- and GDNF-, and CDNF- and GDNF-treated animals (p < 0.05). We also compared total potassium and amphetamine responses between the treatment groups by analyzing average increase in dopamine release during the stimulus responses (Fig. 2b). The results from these analyses supported our findings: Average potassium-evoked dopamine overflow was augmented in MANF-treated rats as compared with the vehicle- and GDNF-treated rats (one-way ANOVA F3,36 = 4.874; p = 0.006; REGWF p < 0.05). Congruently, average amphetamine-evoked dopamine overflow was augmented in MANF group when compared to the vehicle and GDNF groups (one-way ANOVA F3,36 = 3.683; p = 0.021; REGWF p < 0.05).
Three weeks after the surgery, we did not find statistically significant differences between the treatment groups. Figures showing microdialysis results at 3 weeks after the surgery are presented in supplementary material (Fig. 5).
DOPAC concentration in the extracellular fluid decreased during the potassium and amphetamine responses compared to the baseline level in all treatment groups as reported earlier [57, 58] (Fig. 2c, supplementary material Fig. 5b). Similarly, also HVA concentration decreased during the potassium response but increased during the amphetamine response (Fig. 2d, supplementary material Fig. 5c). No statistical differences, however, were observed between the treatment groups in extracellular concentrations of DOPAC or HVA.
Increased Dopamine Turnover in MANF-Treated Rats
To study if NTFs were able to alter dopamine metabolism in the striatum, we determined dopamine turnover by calculating DOPAC/dopamine and HVA/dopamine ratios from the microdialysis samples. One week after the surgery, MANF produced marked increase in dopamine turnover as evaluated by DOPAC/dopamine ratio (Fig. 3a). The ratio was significantly higher in MANF-treated rats as compared with the vehicle- and GDNF-treated rats during the whole experiment (ANOVA for repeated measures 15–270 min: F3,36 = 3.065; p = 0.040; REGWF p < 0.05) as well as in the baseline samples (ANOVA for repeated measures 15–60 min: F3,36 = 3.868; p = 0.017; REGWF p < 0.05). Three weeks after the surgery, there were no differences between the treatment groups in DOPAC/dopamine ratio (supplementary material Fig. 6a). We did not see any significant differences in HVA/dopamine ratio between the treatment groups either 1 or 3 weeks after the surgery (Fig. 3b, supplementary material Fig. 6b).
Dopamine turnover analyzed 1 week after an intrastriatal injection of GDNF, CDNF, MANF, or vehicle. DOPAC/dopamine, HVA/dopamine, and DOPAC/HVA ratios were calculated as ratios of the analyte concentrations in the microdialysis samples. a DOPAC/dopamine turnover was enhanced in MANF-treated rats as compared to the rats treated with vehicle or GDNF during the whole experiment (15–270 min) and during the baseline (15–60 min) (*p < 0.05 MANF vs. VEH and MANF vs. GDNF; REGWF post hoc analysis after ANOVA for repeated measures). b There were no significant changes in HVA/dopamine turnover. c Less DOPAC was formed compared to HVA in GDNF-treated rats as measured by DOPAC/HVA ratio which was significantly smaller in GDNF group than in the other treatment groups during the whole experiment (15–270 min) (#p < 0.05 GDNF vs. VEH, GDNF vs. CDNF and GDNF vs. MANF; REGWF post hoc analysis after ANOVA for repeated measures). The period of potassium and amphetamine responses are depicted under the x-axes. Results are shown as concentration ratios; mean ± SEM; n = 10 in each group
We also calculated the ratio of the metabolites from the microdialysis samples. Unexpectedly, 1 week after the surgery, DOPAC/HVA ratio was significantly reduced in GDNF-treated rats compared to all the other treatment groups during the whole experiment (ANOVA for repeated measures 15–270 min: F3,36 = 7.397; p = 0.001; REGWF p < 0.05) (Fig. 3c). Three weeks after the surgery, DOPAC/HVA ratio was still smaller in GDNF group than in the other groups but the difference did not reach significance (ANOVA for repeated measures 15–270 min: F3,32 = 2.537; p = 0.074) (supplementary material Fig. 6c). The changed DOPAC/HVA ratio suggests that GDNF injection alters the activity of dopamine metabolizing enzymes COMT and MAO.
Increased In Vivo TH Activity in Rats Treated with GDNF
As we found elevated stimulus-evoked release of dopamine in MANF-, but not in GDNF-, treated rats, we wanted to investigate the effect of the NTF injection on in vivo activity of TH. To that end, we inhibited AADC enzyme in the brain with a blood-brain barrier passing inhibitor NSD1015 1 week after the NTF injection. Half an hour after AADC inhibition, we collected tissue samples from the dorsal striatum and quantified accumulated L-DOPA in the samples. In this experiment, the amount of L-DOPA accumulated into the striatum provides a direct measure for L-DOPA production rate, which reflects the in vivo activity of phosphorylated and non-phosphorylated TH in the nigrostriatal pathway [51].
GDNF was able to increase TH activity as the amount of L-DOPA in the treated striatum was increased approximately by 60% as compared with the vehicle (one-way ANOVA F3,22 = 3.780; p = 0.025; REGWF p < 0.05) (Fig. 4a). MANF also tended to enhance the accumulation of L-DOPA (approximately by 50%) while CDNF had no effect. The amount of L-DOPA in treated versus untreated striatum did not differ within the treatment groups when compared with paired two-tailed Student’s t test.
Effect of NTF treatment on the activity of dopamine neurochemistry-regulating enzymes. a In vivo TH activity was assayed 1 week after an intrastriatal injection of GDNF, CDNF, MANF, or vehicle by collecting striatal tissue samples 30 min after the inhibition of aromatic amino acid dopa decarboxylase with NSD1015 (100 mg/kg, i.p.) and analyzing the amount of accumulated L-DOPA in the samples. L-DOPA was synthetized more in rats treated with GDNF as compared to the vehicle-treated rats which indicates increased TH activity in GDNF-injected striata (*p < 0.05 GDNF vs. VEH; one-way ANOVA followed by REGWF post hoc test). b–d Due to significantly reduced DOPAC/HVA ratio in GDNF-treated rats seen in the microdialysis experiments, the effect of GDNF on COMT, MAO-A, and MAO-B activity was determined from striatal tissue samples collected 1 week after an intrastriatal injection of GDNF or vehicle. b GDNF significantly increased total COMT activity in the treated striatum as compared to the vehicle-treated rats (**p < 0.001; unpaired two-tailed Student’s t test) as well as compared to the untreated side (#p < 0.001; paired two-tailed Student’s t test). c GDNF significantly reduced MAO-A activity in the treated striatum as compared to the vehicle-treated rats (* p = 0.011; unpaired two-tailed Student’s t test). d MAO-B activity was also reduced in rats treated with GDNF but not significantly. Results are shown as mean ± SEM; in TH activity experiment, n = 6–7 in each group; in COMT and MAO activity experiments, n = 7–8
Increased COMT Activity and Reduced MAO Activity in Rats Treated with GDNF
Because of the significantly reduced DOPAC/HVA ratio in GDNF-treated rats, we decided to assess the effect of GDNF on dopamine-metabolizing enzymes COMT, MAO-A, and MAO-B. To study this, GDNF or vehicle was unilaterally injected into the dorsal striatum and 1 week later, striatal tissue samples were collected for ex vivo enzyme activity assays.
GDNF increased the total activity of COMT by 155% in the treated striatum when compared to the vehicle-treated rats (unpaired two-tailed Student’s t test t(13) = − 5.159; p < 0.001) and by 170% when compared to the untreated striatum (paired two-tailed Student’s t test t(7) = 6.041; p < 0.001) (Fig. 4b). GDNF also reduced MAO-A activity in the treated striatum by 27% when compared to the vehicle-treated controls (unpaired two-tailed Student’s t test t(14) = 2.944; p = 0.011) (Fig. 4c). GDNF had a modest reducing effect on MAO-B activity (approximately 18% as compared to the vehicle-treated controls), but the difference remained insignificant (unpaired two-tailed Student’s t test t(14) = 1.689; p = 0.113) (Fig. 4d).
Discussion
The main findings in this study were as follows: (i) Intrastriatal injection of MANF elevated stimulus-evoked release of dopamine in the striatum 1 week after the injection. Elevated dopamine release was accompanied by enhanced DOPAC/dopamine turnover in MANF-treated rats. (ii) In GDNF-treated rats, stimulus-evoked release of dopamine was not changed although striatal TH activity was increased. At the same time, DOPAC/HVA ratio was decreased apparently due to increased COMT activity and decreased MAO-A activity.
To be able to assess different presynaptic release mechanisms, we utilized a microdialysis protocol with two distinct stimuli (high concentration of K+ and amphetamine) to evoke dopamine release from nigrostriatal nerve endings. We saw smaller differences in dopamine overflow between the treatment groups during the amphetamine response than during the potassium response (Fig. 2a). High concentration of potassium depolarizes nerve terminals and causes fusion of vesicles close to the presynaptic membrane in a calcium-dependent manner [59]. This pool of presynaptic dopamine is considered to be readily releasable. Amphetamine, on the contrary, is known to deplete vesicular stores of dopamine and reverse the function of DAT causing calcium independent release of dopamine [56, 59]. Thus, amphetamine stimulus gives an estimate of the total amount of dopamine stored in nerve terminals. Our results may indicate that MANF can enhance the dynamics of calcium mediated membrane fusion of presynaptic dopamine vesicles or increase the proportion of readily releasable vesicles as demonstrated by elevated potassium-evoked release of dopamine.
One week after the NTF injection, stimulus-evoked release of dopamine was elevated in MANF-treated rats suggesting increased sprouting of dopaminergic fibers (Fig. 2a). This may not be the case, however, since Voutilainen et al. showed no effect of 2-week intrastriatal infusion of MANF on TH-immunoreactivity in the substantia nigra or striatum in intact rats [15]. Thus, it is more likely that MANF enhances dopaminergic neurotransmission through presynaptic storage or release mechanisms rather than through sprouting. For example, GDNF has been proposed to facilitate synaptic transmission by modulating the quantal size of neurotransmitter release [19], potentiating Ca2+ influx [22, 60], and inhibiting A-type K+-channels [21], thereby potentiating excitability of neurons. It is possible that MANF has same type of modulatory effects on the function of nerve terminals although its possible effects on ion channels still remain to be clarified.
Contrary to the previous studies, the stimulus-evoked release of dopamine was not increased in GDNF-treated animals [24,25,26, 28, 29]. The previous studies were conducted under general anesthesia. Anesthetics are known to have marked effects on neurotransmission which can explain the differing results from the present study [30, 31]. Similarly to our results, by using freely-moving rats with only a brief metofane anesthesia at the beginning of microdialysis experiment, Xu and Dluzen did not see significant differences between GDNF- and vehicle-injected rats [61]. In a microdialysis study with freely-moving mice, potassium-evoked release of dopamine did not differ between wild-type and MEN2B mice that have constantly active RET [62]. These observations are in line with our results. DAT activity has been shown to be markedly increased in MEN2B mice and in GDNF hypermorphic mice overexpressing GDNF [62, 63]. Therefore, it can be hypothesized that in the present study, increased DAT activity after GDNF treatment results in enhanced clearance of extracellular dopamine after potassium-stimulus and thus nullifies the dopaminergic transmission enhancing effect of GDNF. In addition, during amphetamine response, when dopamine reuptake through DAT and metabolism through MAO are inhibited, the role of COMT in dopamine turnover is pronounced. As GDNF was shown to increase COMT activity, it can be speculated that amphetamine-evoked dopamine release was diminished in GDNF-treated rats due to increased metabolism through COMT.
Baseline concentration of dopamine and its metabolites remained unchanged between the treatment groups at 1 and 3 weeks after the NTF injection (Table 1). This result is in line with earlier microdialysis experiments: there were no differences in the basal extracellular dopamine concentration between GDNF- and vehicle-treated rats [24, 61] or between MEN2B and wild-type mice [62]. The unchanged baseline concentrations may result from effective homeostatic mechanisms after NTF treatments, including enhanced uptake of dopamine through DAT. It is also possible that dopamine is stored more in terminal vesicles in NTF-treated animals, while the tonic release of dopamine remains unchanged during the baseline.
To study if the elevated release of dopamine in MANF-injected rats was due to enhanced synthesis of dopamine, we determined the effect of the NTFs on in vivo TH activity. Because of the fact that unilaterally injected NTFs have bilateral effects [42, 64, 65], we compared the amount of L-DOPA only in the treated striata between the treatment groups. We saw significantly increased TH activity in GDNF-treated rats, measured as accumulated striatal L-DOPA following inhibition of AADC [51] (Fig. 4a). MANF also seemed to have an increasing effect on TH activity, but this effect was not significant. Thus, TH activity cannot solely explain the significant elevation in the stimulus-evoked dopamine release seen in MANF-treated rats. The effect of GDNF on TH activity, on the other hand, is in line with an earlier observation according to which continuous RET activation in MEN2B mice increases in vivo TH activity [62]. Although exogenous GDNF has been shown to downregulate the total expression of TH in dopamine neurons, it can also increase the phosphorylation of TH and consequently the activity of the enzyme [28, 42,43,44,45,46]. Thus, the differences in TH activity between the treatment groups can be due to different ability of NTFs to enhance the phosphorylation of TH. The downregulation of TH after GDNF administration may be a compensatory response to its increased phosphorylation and activity. Increased TH activity in GDNF-treated rats may also be due to decreased amount of dopamine in nerve terminals which might affect the activity of TH through feed-back mechanisms as speculated by Georgievska et al. [44].
DOPAC/dopamine ratio was measured from the microdialysis samples as an indicator of dopamine metabolism. The turnover of dopamine into DOPAC was significantly increased in MANF group as compared to vehicle and GDNF groups 1 week after the NTF injection (Fig. 3a). Moreover, HVA/dopamine turnover seemed to be increased in MANF group but the differences were not statistically significant (Fig. 3b). Increased DOPAC/dopamine ratio can be considered a sign of enhanced dopaminergic neurotransmission [67, 68]. In earlier studies, DOPAC/dopamine and HVA/dopamine ratios in striatal and nigral tissue samples were increased in GDNF-treated animals [66,67,68,69], but Hebert et al. reported unchanged dopamine turnover in GDNF-treated rats [24]. Apart from enhanced dopaminergic neurotransmission, the increased DOPAC/dopamine turnover after MANF treatment can be a consequence of augmented tonic release of dopamine outside the stimulus responses. As the metabolism of dopamine is efficient, increased amount of extracellular dopamine might lead to the higher turnover ratio.
Interestingly, we saw a significantly reduced DOPAC/HVA ratio in GDNF-treated rats at 1 week after the NTF injection (Fig. 3c). To further elucidate this phenomenon, we tested the possible effect of GDNF on the activity of the dopamine-metabolizing enzymes COMT, MAO-A, and MAO-B. GDNF significantly increased total COMT activity and decreased MAO-A activity in the striatum when compared to the vehicle, giving a logical explanation for the reduced DOPAC/HVA ratio (Fig. 4b, c). Helkamaa et al. have demonstrated that LPS-induced microglia activation results in increased COMT activity in the rat brain [70]. In the present study, however, microglia activation due to surgical procedures cannot explain the increased activity of COMT in GDNF-injected rats, because the vehicle injection did not cause any changes in total COMT activity when compared to the non-injected side (Fig. 4b). Consequently, GDNF seems to have a direct increasing effect on COMT activity or expression or both.
One possible factor behind the divergent effects of the NTFs on dopamine neurochemistry seen in our experiments can be their different diffusion properties within brain parenchyma. Volume of distribution of GDNF in the brain is limited by its high affinity binding to heparan sulfate proteoglycans in the extracellular matrix and cell surfaces [71]. CDNF and especially MANF, on the other hand, have shown to have better diffusion properties in the rat brain when compared to GDNF [14, 15]. Thus, efficient distribution of MANF in the striatum may explain its more pronounced effect on dopamine release. Another explanation for the divergent effects can be different production methods of the NTFs. GDNF was produced in E. coli bacterial cells, CDNF in Sf9 insect cells and MANF in CHO mammalian cells. Proteins produced in bacterial cells are not glycosylated after the translation whereas proteins produced in insect or mammalian cells can be glycosylated. Glycosylation may affect the activity and diffusion properties of the proteins in the brain. However, non-glycosylated GDNF produced in E. coli has been shown to have full biological activity [8]. According to our mass spectrometer analysis neither CDNF nor MANF used here were glycosylated making them comparable with the GDNF of bacterial origin. Nevertheless, it can be speculated that recombinant NTFs produced in mammalian cells may have stronger biological activity than NTFs produced in other cell lines [72].
MANF and CDNF have been proposed to work under the condition of ER stress or inflammation [38,39,40,41]. The microdialysis protocol used here indeed causes ER stress and inflammation due to mechanical damage around the sampling site. The damage also results in local degeneration of nerve terminals, edema, changes in bloodstream, and gliosis around the probe membrane [73]. Repeated insertion and removal of the probe may affect the results of the second microdialysis due to pathological changes around the perfusion area; glial scar forms a diffusion barrier for the analytes which may explain the general decline in the baseline analyte concentrations 3 versus 1 week after the NTF injection (Table 1). The activation of MANF and CDNF in ER stress conditions might be a part of the explanation why they had different effects than GDNF in this study setting. In addition, the mechanical damage caused by the implantation of the guide cannula right after the NTF injection causes an inevitable disruption of the blood-brain barrier. Due to this disruption, antibodies neutralizing exogenous NTFs may invade into the brain abolishing part of the effect of the NTFs and causing unexpected variation to the results.
To this day, the cornerstone of the treatment of PD has been pharmacological substitution of striatal dopamine with initially good efficacy, but no effect on disease progression. NTFs are regarded as the first potential disease modifying therapy for PD as they are able to halt the progression of neurodegeneration and restore aberrant neuronal function in various experimental settings. However, clinical trials with NTFs show conflicting results. Therefore, it is important to better understand the effects of NTFs on dopaminergic functions of non-lesioned brain. Our current results reveal divergent biological effects of exogenously administrated GDNF, CDNF, and MANF. MANF is able to potentiate stimulus-evoked dopaminergic neurotransmission and enhance dopamine turnover in the brain of freely-moving rats. GDNF, on the other hand, increases the activity of TH and COMT and decreases the activity of MAO-A. This study gives an insight into the long-lasting changes in dopamine synthesis, release and metabolism after a single intrastriatal NTF injection which is highly relevant information for the development of novel therapeutic strategies for neurodegenerative diseases. However, further studies are needed to clarify the cellular mechanisms by which the NTFs produce their effects on neuronal homeostasis seen in this study.
References
Poewe W, Seppi K, Tanner CM, Halliday GM, Brundin P, Volkmann J, Schrag A-E, Lang AE (2017) Parkinson’s disease. Nat Rev Dis Prim 3:1–21. https://doi.org/10.1038/nrdp.2017.13
Dauer W, Przedborski S (2003) Parkinson’s disease: mechanisms and models. Neuron 39(6):889–909. https://doi.org/10.1016/S0896-6273(03)00568-3
Kalia LV, Lang AE (2015) Parkinson’s disease. Lancet 386(9996):896–912. https://doi.org/10.1016/S0140-6736(14)61393-3
Huang EJ, Reichardt LF (2001) Neurotrophins: roles in neuronal development and function. Annu Rev Neurosci 24(1):677–736. https://doi.org/10.1146/annurev.neuro.24.1.677
Airaksinen MS, Saarma M (2002) The GDNF family: signalling, biological functions and therapeutic value. Nat Rev Neurosci 3(5):383–394. https://doi.org/10.1038/nrn812
Lindholm P, Saarma M (2010) Novel CDNF/MANF family of neurotrophic factors. Dev Neurobiol 70:360–371. https://doi.org/10.1002/dneu.20760
Aron L, Klein R (2011) Repairing the parkinsonian brain with neurotrophic factors. Trends Neurosci 34(2):88–100. https://doi.org/10.1016/j.tins.2010.11.001
Lin L-FH, Doherty DH, Lile JD, Bektesh S, Collins F (1993) GDNF: a glial cell line-derived neurotrophic factor for midbrain dopaminergic neurons. Science 260(5111):1130–1132. https://doi.org/10.1126/science.8493557
Hoffer BJ, Hoffman A, Bowenkamp K, Huettl P, Hudson J, Martin D, Lin L-FH, Gerhardt GA (1994) Glial cell line-derived neurotrophic factor reverses toxin-induced injury to midbrain dopaminergic neurons in vivo. Neurosci Lett 182(1):107–111. https://doi.org/10.1016/0304-3940(94)90218-6
Tomac A, Lindqvist E, Lin L-FH, Ögren SO, Young D, Hoffer BJ, Olson L (1995) Protection and repair of the nigrostriatal dopaminergic system by GDNF in vivo. Nature 373(6512):335–339. https://doi.org/10.1038/373335a0
Gash DM, Zhang Z, Ovadia A, Cass WA, Yi A, Simmerman L, Russell D, Martin D et al (1996) Functional recovery in parkinsonian monkeys treated with GDNF. Nature 380(6571):252–255. https://doi.org/10.1038/380252a0
Petrova PS, Raibekas A, Pevsner J, Vigo N, Anafi M, Moore MK, Peaire AE, Shridhar V et al (2003) MANF: a new mesencephalic, astrocyte-derived neurotrophic factor with selectivity for dopaminergic neurons. J Mol Neurosci 20(2):173–187. https://doi.org/10.1385/JMN:20:2:173
Lindholm P, Voutilainen MH, Laurén J, Peränen J, Leppänen VM, Andressoo J-O, Lindahl M, Janhunen S et al (2007) Novel neurotrophic factor CDNF protects and rescues midbrain dopamine neurons in vivo. Nature 448(7149):73–77. https://doi.org/10.1038/nature05957
Voutilainen MH, Bäck S, Pörsti E, Toppinen L, Lindgren L, Lindholm P, Peränen J, Saarma M et al (2009) Mesencephalic astrocyte-derived neurotrophic factor is neurorestorative in rat model of Parkinson’s disease. J Neurosci 29(30):9651–9659. https://doi.org/10.1523/JNEUROSCI.0833-09.2009
Voutilainen MH, Bäck S, Peränen J, Lindholm P, Raasmaja A, Männistö PT, Saarma M, Tuominen RK (2011) Chronic infusion of CDNF prevents 6-OHDA-induced deficits in a rat model of Parkinson’s disease. Exp Neurol 228(1):99–108. https://doi.org/10.1016/j.expneurol.2010.12.013
Airavaara M, Harvey BK, Voutilainen MH, Shen H, Chou J, Lindholm P, Lindahl M, Tuominen RK et al (2012) CDNF protects the nigrostriatal dopamine system and promotes recovery after MPTP treatment in mice. Cell Transplant 21(6):1213–1223. https://doi.org/10.3727/096368911X600948
Bäck S, Peränen J, Galli E, Pulkkila P, Lonka-Nevalaita L, Tamminen T, Voutilainen MH, Raasmaja A et al (2013) Gene therapy with AAV2-CDNF provides functional benefits in a rat model of Parkinson’s disease. Brain Behav 3(2):75–88. https://doi.org/10.1002/brb3.117
Garea-Rodríguez E, Eesmaa A, Lindholm P, Schlumbohm C, König J, Meller B, Krieglstein K, Helms G et al (2016) Comparative analysis of the effects of neurotrophic factors CDNF and GDNF in a nonhuman primate model of Parkinson’s disease. PLoS One 11(2):e0149776. https://doi.org/10.1371/journal.pone.0149776
Pothos EN, Davila V, Sulzer D (1998) Presynaptic recording of quanta from midbrain dopamine neurons and modulation of the quantal size. J Neurosci 18(11):4106–4118
Feng L, Wang CY, Jiang H, Oho C, Mizuno K, Dugich-Djordjevic M, Lu B (1999) Differential effects of GDNF and BDNF on cultured ventral mesencephalic neurons. Mol Brain Res 66(1-2):62–70. https://doi.org/10.1016/S0169-328X(99)00015-7
Yang F, Feng L, Zheng F, Johnson SW, Du J, Shen L, Wu CP, Lu B (2001) GDNF acutely modulates excitability and A-type K+ channels in midbrain dopaminergic neurons. Nat Neurosci 4(11):1071–1078. https://doi.org/10.1038/nn734
Wang J, Chen G, Lu B, Wu CP (2003) GDNF acutely potentiates Ca2+ channels and excitatory synaptic transmission in midbrain dopaminergic neurons. Neurosignals 12(2):78–88. https://doi.org/10.1159/000071817
Gash DM, Zhang Z, Cass WA, Ovadia A, Simmerman L, Martin D, Russell D, Collins F et al (1995) Morphological and functional effects of intranigrally administered GDNF in normal rhesus monkeys. J Comp Neurol 363(3):345–358. https://doi.org/10.1002/cne.903630302
Hebert MA, Van Horne CG, Hoffer BJ, Gerhardt GA (1996) Functional effects of GDNF in normal rat striatum: presynaptic studies using in vivo electrochemistry and microdialysis. J Pharmacol Exp Ther 279(3):1181–1190
Hebert MA, Gerhardt GA (1997) Behavioral and neurochemical effects of intranigral administration of glial cell line-derived neurotrophic factor on aged Fischer 344 rats. J Pharmacol Exp Ther 282(2):760–768
Cass WA, Walker DJ, Manning MW (1999) Augmented methamphetamine-induced overflow of striatal dopamine 1 day after GDNF administration. Brain Res 827(1-2):104–112. https://doi.org/10.1016/S0006-8993(99)01314-1
Grondin R, Cass WA, Zhang Z, Stanford JA, Gash DM, Gerhardt GA (2003) Glial cell line-derived neurotrophic factor increases stimulus-evoked dopamine release and motor speed in aged rhesus monkeys. J Neurosci 23:1974–1980
Salvatore MF, Zhang JL, Large DM, Wilson PE, Gash CR, Thomas TC, Haycock JW, Bing G et al (2004) Striatal GDNF administration increases tyrosine hydroxylase phosphorylation in the rat striatum and substantia nigra. J Neurochem 90(1):245–254. https://doi.org/10.1111/j.1471-4159.2004.02496.x
Cass WA, Peters LE (2010) Neurturin effects on nigrostriatal dopamine release and content: Comparison with GDNF. Neurochem Res 35(5):727–734. https://doi.org/10.1007/s11064-010-0128-0
Müller CP, Pum ME, Amato D, Schüttler J, Huston JP, De Souza Silva MA (2011) The in vivo neurochemistry of the brain during general anesthesia. J Neurochem 119(3):419–446. https://doi.org/10.1111/j.1471-4159.2011.07445.x
Marinelli M, McCutcheon JE (2014) Heterogeneity of dopamine neuron activity across traits and states. Neuroscience 282:176–197. https://doi.org/10.1016/j.neuroscience.2014.07.034
Parkash V, Lindholm P, Peränen J, Kalkkinen N, Oksanen E, Saarma M, Leppänen VM, Goldman A (2009) The structure of the conserved neurotrophic factors MANF and CDNF explains why they are bifunctional. Protein Eng Des Sel 22(4):233–241. https://doi.org/10.1093/protein/gzn080
Hellman M, Arumäe U, Yu L-Y, Lindholm P, Peränen J, Saarma M, Permi P (2011) Mesencephalic astrocyte-derived neurotrophic factor (MANF) has a unique mechanism to rescue apoptotic neurons. J Biol Chem 286(4):2675–2680. https://doi.org/10.1074/jbc.M110.146738
Lindahl M, Saarma M, Lindholm P (2017) Unconventional neurotrophic factors CDNF and MANF: Structure, physiological functions and therapeutic potential. Neurobiol Dis 97(Pt B):90–102. https://doi.org/10.1016/j.nbd.2016.07.009
Apostolou A, Shen Y, Liang Y, Luo J, Fang S (2008) Armet, a UPR-upregulated protein, inhibits cell proliferation and ER stress-induced cell death. Exp Cell Res 314(13):2454–2467. https://doi.org/10.1016/j.yexcr.2008.05.001
Tadimalla A, Belmont PJ, Thuerauf DJ, Glassy MS, Martindale JJ, Gude N, Sussman MA, Glembotski CC (2008) Mesencephalic astrocyte-derived neurotrophic factor is an ischemia-inducible secreted endoplasmic reticulum stress response protein in the heart. Circ Res 103(11):1249–1258. https://doi.org/10.1161/CIRCRESAHA.108.180679
Cheng L, Zhao H, Zhang W, Liu B, Liu Y, Guo Y-J, Nie L (2013) Overexpression of conserved dopamine neurotrophic factor (CDNF) in astrocytes alleviates endoplasmic reticulum stress-induced cell damage and inflammatory cytokine secretion. Biochem Biophys Res Commun 435(1):34–39. https://doi.org/10.1016/j.bbrc.2013.04.029
Zhao H, Liu Y, Cheng L, Liu B, Zhang W, Guo Y-J, Nie L (2013) Mesencephalic astrocyte-derived neurotrophic factor inhibits oxygen–glucose deprivation-induced cell damage and inflammation by suppressing endoplasmic reticulum stress in rat primary astrocytes. J Mol Neurosci 51(3):671–678. https://doi.org/10.1007/s12031-013-0042-4
Chen L, Feng L, Wang X, Du J, Chen Y, Yang W, Zhou C, Cheng L et al (2015) Mesencephalic astrocyte-derived neurotrophic factor is involved in inflammation by negatively regulating the NF-kappaB pathway. Sci Rep 5(1). https://doi.org/10.1038/srep08133
Voutilainen MH, Arumäe U, Airavaara M, Saarma M (2015) Therapeutic potential of the endoplasmic reticulum located and secreted CDNF/MANF family of neurotrophic factors in Parkinson’s disease. FEBS Lett 589(24PartA):3739–3748. https://doi.org/10.1016/j.febslet.2015.09.031
Voutilainen MH, De Lorenzo F, Stepanova P, Bäck S, Yu L-Y, Lindholm P, Pörsti E, Saarma M et al (2017) Evidence for an additive neurorestorative effect of simultaneously administered CDNF and GDNF in hemiparkinsonian rats: Implications for different mechanism of action. eNeuro 4:ENEURO.0117–ENEU16.2017. https://doi.org/10.1523/ENEURO.0117-16.2017
Salvatore MF, Gerhardt GA, Dayton RD, Klein RL, Stanford JA (2009) Bilateral effects of unilateral GDNF administration on dopamine- and GABA-regulating proteins in the rat nigrostriatal system. Exp Neurol 219(1):197–207. https://doi.org/10.1016/j.expneurol.2009.05.013
Rosenblad C, Georgievska B, Kirik D (2003) Long-term striatal overexpression of GDNF selectively downregulates tyrosine hydroxylase in the intact nigrostriatal dopamine system. Eur J Neurosci 17(2):260–270. https://doi.org/10.1046/j.1460-9568.2003.02456.x
Georgievska B, Kirik D, Björklund A (2004) Overexpression of glial cell line-derived neurotrophic factor using a lentiviral vector induces time- and dose-dependent downregulation of tyrosine hydroxylase in the intact nigrostriatal dopamine system. J Neurosci 24(29):6437–6445. https://doi.org/10.1523/JNEUROSCI.1122-04.2004
Salvatore MF, Waymire JC, Haycock JW (2001) Depolarization-stimulated catecholamine biosynthesis: Involvement of protein kinases and tyrosine hydroxylase phosphorylation sites in situ. J Neurochem 79(2):349–360. https://doi.org/10.1046/j.1471-4159.2001.00593.x
Kobori N, Waymire JC, Haycock JW, Clifton GL, Dash PK (2004) Enhancement of tyrosine hydroxylase phosphorylation and activity by glial cell line-derived neurotrophic factor. J Biol Chem 279(3):2182–2191. https://doi.org/10.1074/jbc.M310734200
Paxinos G, Watson C (1998) The rat brain in stereotaxic coordinates, 4th edn. Academic Press, San Diego
Glick SD, Dong N, Keller RW, Carlson JN (1994) Estimating extracellular concentrations of dopamine and 3,4-dihydroxyphenylacetic acid in nucleus accumbens and striatum using microdialysis: Relationships between in vitro and in vivo recoveries. J Neurochem 62(5):2017–2021. https://doi.org/10.1046/j.1471-4159.1994.62052017.x
Airavaara M, Mijatovic J, Vihavainen T, Piepponen TP, Saarma M, Ahtee L (2006) In heterozygous GDNF knockout mice the response of striatal dopaminergic system to acute morphine is altered. Synapse 59(6):321–329. https://doi.org/10.1002/syn.20245
Käenmäki M, Tammimäki A, Myöhänen T, Pakarinen K, Amberg C, Karayiorgou M, Gogos JA, Männistö PT (2010) Quantitative role of COMT in dopamine clearance in the prefrontal cortex of freely moving mice. J Neurochem 114(6):1745–1755. https://doi.org/10.1111/j.1471-4159.2010.06889.x
Carlsson A, Davis JN, Kehr W, Lindqvist M, Atack CV (1972) Simultaneous measurement of tyrosine and tryptophan hydroxylase activities in brain in vivo using an inhibitor of the aromatic amino acid decarboxylase. Naunyn Schmiedeberg's Arch Pharmacol 275(2):153–168. https://doi.org/10.1007/BF00508904
Kankaanpää A, Meririnne E, Ariniemi K, Seppälä T (2001) Oxalic acid stabilizes dopamine, serotonin, and their metabolites in automated liquid chromatography with electrochemical detection. J Chromatogr B Biomed Sci Appl 753(2):413–419. https://doi.org/10.1016/S0378-4347(00)00553-3
Valros A, Palander P, Heinonen M, Munsterhjelm C, Brunberg E, Keeling L, Piepponen TP (2015) Evidence for a link between tail biting and central monoamine metabolism in pigs (Sus scrofa domestica). Physiol Behav 143:151–157. https://doi.org/10.1016/j.physbeh.2015.02.049
Schendzielorz N, Männistö PT, Karayiorgou M, Gogos JA, Raasmaja A (2012) A transient inhibition and permanent lack of catechol-O-methyltransferase have minor effects on feeding pattern of female rodents. Basic Clin Pharmacol Toxicol 110(4):307–313. https://doi.org/10.1111/j.1742-7843.2011.00783.x
Smith SB, Reenilä I, Männistö PT, Slade GD, Maixner W, Diatchenko L, Nackley AG (2014) Epistasis between polymorphisms in COMT, ESR1, and GCH1 influences COMT enzyme activity and pain. Pain 155(11):2390–2399. https://doi.org/10.1016/j.pain.2014.09.009
Sulzer D (2011) How addictive drugs disrupt presynaptic dopamine neurotransmission. Neuron 69(4):628–649. https://doi.org/10.1016/j.neuron.2011.02.010
Zetterström T, Sharp T, Collin A, Ungerstedt U (1988) In vivo measurement of extracellular dopamine and DOPAC in rat striatum after various dopamine-releasing drugs; implications for the origin of extracellular DOPAC. Eur J Pharmacol 148(3):327–334. https://doi.org/10.1016/0014-2999(88)90110-0
Kuczenski R, Segal D (1989) Concomitant characterization of behavioral and striatal neurotransmitter response to amphetamine using in vivo microdialysis. J Neurosci 9(6):2051–2065
Westerink BHC, Hofsteede RM, Tuntler J, de Vries JB (1989) Use of calcium antagonism for the characterization of drug-evoked dopamine release from the brain of conscious rats determined by microdialysis. J Neurochem 52(3):722–729. https://doi.org/10.1111/j.1471-4159.1989.tb02514.x
Wang C-Y, Yang F, He X, Chow A, Du J, Russell JT, Lu B (2001) Ca2+ binding protein frequenin mediates GDNF-induced potentiation of Ca2+ channels and transmitter release. Neuron 32(1):99–112. https://doi.org/10.1016/S0896-6273(01)00434-2
Xu K, Dluzen DE (2000) The effect of GDNF on nigrostriatal dopaminergic function in response to a two-pulse K+ stimulation. Exp Neurol 166(2):450–457. https://doi.org/10.1006/exnr.2000.7515
Mijatovic J, Patrikainen O, Yavich L, Airavaara M, Ahtee L, Saarma M, Piepponen TP (2008) Characterization of the striatal dopaminergic neurotransmission in MEN2B mice with elevated cerebral tissue dopamine. J Neurochem 105(5):1716–1725. https://doi.org/10.1111/j.1471-4159.2008.05265.x
Kumar A, Kopra J, Varendi K, Porokuokka LL, Panhelainen A, Kuure S, Marshall P, Karalija N et al (2015) GDNF overexpression from the native locus reveals its role in the nigrostriatal dopaminergic system function. PLoS Genet 11(12):e1005710. https://doi.org/10.1371/journal.pgen.1005710
Stanford JA, Salvatore MF, Joyce BM, Zhang H, Gash DM, Gerhardt GA (2007) Bilateral effects of unilateral intrastriatal GDNF on locomotor-excited and nonlocomotor-related striatal neurons in aged F344 rats. Neurobiol Aging 28(1):156–165. https://doi.org/10.1016/j.neurobiolaging.2005.10.015
Garbayo E, Ansorena E, Lana H, Carmona-Abellán MM, Marcilla I, Lanciego JL, Luquin MR, Blanco-Prieto MJ (2016) Brain delivery of microencapsulated GDNF induces functional and structural recovery in parkinsonian monkeys. Biomaterials 110:11–23. https://doi.org/10.1016/j.biomaterials.2016.09.015
Martin D, Miller G, Cullen T, Fischer N, Dix D, Russell D (1996) Intranigral or intrastriatal injections of GDNF: effects on monoamine levels and behavior in rats. Eur J Pharmacol 317(2-3):247–256. https://doi.org/10.1016/S0014-2999(96)00756-X
Hadaczek P, Johnston L, Forsayeth J, Bankiewicz KS (2010) Pharmacokinetics and bioactivity of glial cell line-derived factor (GDNF) and neurturin (NTN) infused into the rat brain. Neuropharmacology 58(7):1114–1121. https://doi.org/10.1016/j.neuropharm.2010.02.002
Hudson J, Granholm A-C, Gerhardt GA, Henry MA, Hoffman A, Biddle P, Leela NS, Mackerlova L et al (1995) Glial cell line-derived neurotrophic factor augments midbrain dopaminergic circuits in vivo. Brain Res Bull 36(5):425–432. https://doi.org/10.1016/0361-9230(94)00224-O
Horger BA, Nishimura MC, Armanini MP, Wang L-C, Poulsen KT, Rosenblad C, Kirik D, Moffat B et al (1998) Neurturin exerts potent actions on survival and function of midbrain dopaminergic neurons. J Neurosci 18(13):4929–4937
Helkamaa T, Reenilä I, Tuominen RK, Soinila S, Väänänen A, Tilgmann C, Rauhala P (2007) Increased catechol-O-methyltransferase activity and protein expression in OX-42-positive cells in the substantia nigra after lipopolysaccharide microinfusion. Neurochem Int 51(6-7):412–423. https://doi.org/10.1016/j.neuint.2007.04.020
Hamilton JF, Morrison PF, Chen MY, Harvey-White J, Pernaute RS, Phillips H, Oldfield E, Bankiewicz KS (2001) Heparin coinfusion during convection-enhanced delivery (CED) increases the distribution of the glial-derived neurotrophic factor (GDNF) ligand family in rat striatum and enhances the pharmacological activity of neurturin. Exp Neurol 168(1):155–161. https://doi.org/10.1006/exnr.2000.7571
Andressoo J-O, Saarma M (2008) Signalling mechanisms underlying development and maintenance of dopamine neurons. Curr Opin Neurobiol 18(3):297–306. https://doi.org/10.1016/j.conb.2008.07.005
Benveniste H, Hansen AJ (1991) Practical aspects of using microdialysis for determination of brain interstitial concentrations. In: Robinson TE, Justice Jr JB (eds) Microdialysis in the neurosciences, 1st edn. Elsevier Science Publishers, Amsterdam, pp. 81–100. https://doi.org/10.1016/B978-0-444-81194-3.50009-5
Acknowledgements
We are grateful to Kati Rautio, Marjo Vaha and Liisa Lappalainen for their excellent technical assistance with the experimental procedures.
Funding
This study was funded by a grant from the Academy of Finland (grant no. 253840) and by the Tekes Large Strategic Research Opening 3i Regeneration (no. 40395/13).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All animal experiments were in accordance with the directive of the European Parliament and of the Council on the protection of animals used for scientific purposes (Directive 2010/63/EU of the European Parliament and of the Council). Experiments were approved by the National Animal Experiment Board of Finland (ESLH-2009-05234/Ym-23 and ESAVI/198/04.10.07/2014).
Conflict of Interest
M.S., R.K.T., and M.H.V. are inventors in CDNF- and MANF-related patent applications which are owned by Herantis Pharma Plc. M.S. is a founder of Herantis Pharma Plc and a member of its Scientific Advisory Board.
Electronic supplementary material
Supplementary Figure 5
Extracellular striatal concentrations of dopamine and its main metabolites 3,4-dihydroxyphenylacetic acid (DOPAC) and homovanillic acid (HVA) measured using brain microdialysis three weeks after an intrastriatal injection of GDNF, CDNF, MANF or vehicle. (a) During the second microdialysis experiment at three weeks after the surgery there were no more statistically significant differences between the treatment groups in stimulus-evoked release of dopamine although potassium response (75-135 min) still tended to be augmented in MANF-treated rats (p = 0.084; ANOVA for repeated measures). (b, c) NTFs did not have significant effects on extracellular DOPAC or HVA concentration at three weeks after the injection. The period when high-potassium and amphetamine perfusion solutions were pumped (K+ and Amphet. administration) (a) and the period of potassium and amphetamine responses (a-c) are depicted under the x-axes. Results are shown as % of baseline value (=100%); mean ± SEM; n = 8-10 in each group (GIF 74 kb)
Supplementary Figure 6
Dopamine turnover analyzed three weeks after an intrastriatal injection of GDNF, CDNF, MANF or vehicle. DOPAC/dopamine, HVA/dopamine and DOPAC/HVA ratios were calculated as ratios of the analyte concentrations in the microdialysis samples. (a) There were no more significant differences between the treatment groups in DOPAC/dopamine turnover at three weeks after the surgery. (b) There were no significant changes in HVA/dopamine turnover either. (c) Three weeks after the surgery DOPAC/HVA ratio was still reduced in rats treated with GDNF although the difference was not statistically significant anymore (p = 0.074; ANOVA for repeated measures). The period of potassium and amphetamine responses are depicted under the x-axes. Results are shown as concentration ratios; mean ± SEM; n = 8-10 in each group (GIF 105 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Renko, JM., Bäck, S., Voutilainen, M.H. et al. Mesencephalic Astrocyte-Derived Neurotrophic Factor (MANF) Elevates Stimulus-Evoked Release of Dopamine in Freely-Moving Rats. Mol Neurobiol 55, 6755–6768 (2018). https://doi.org/10.1007/s12035-018-0872-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-018-0872-8