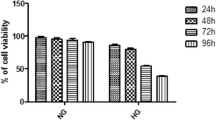
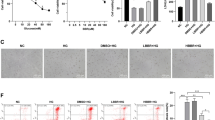
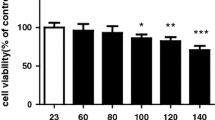
Abstract
Hyperglycemia as the major hallmark of diabetic neuropathy severely limited its therapeutic efficiency. Evidences have revealed that selenium (Se) as an essential trace element could effectively reduce the risk of neurological diseases. In the present study, 3,3′-diselenodipropionic acid (DSePA), a derivative of selenocystine, was employed to investigate its protective effect against high glucose-induced neurotoxicity in PC12 cells and evaluate the underlying mechanism. The results suggested that high glucose showed significant cytotoxicity through launching mitochondria-mediated apoptosis in PC12 cells, accompanied by poly (ADP-ribose) polymerase (PARP) cleavage, caspase activation, and mitochondrial dysfunction. Moreover, high glucose also triggered DNA damage and dysregulation of MAPKs and AKT pathways through reactive oxygen species (ROS) overproduction. p53 RNA interference partially suppressed high glucose-induced cytotoxicity and apoptosis, indicating the role of p53 in high glucose-induced signal. However, DSePA pretreatment effectively attenuated high glucose-induced cytotoxicity, inhibited the mitochondrial dysfunction through regulation of Bcl-2 family, and ultimately reversed high glucose-induced apoptotic cell death in PC12 cells. Attenuation of caspase activation, PARP cleavage, DNA damage, and ROS accumulation all confirmed its protective effects. Moreover, DSePA markedly alleviated the dysregulation of AKT and MAPKs pathways induced by high glucose. Our findings revealed that the strategy of using DSePA to antagonize high glucose-induced neurotoxicity may be a highly effective strategy in combating high glucose-mediated neurological diseases.






Similar content being viewed by others
References
Diogo CV, Suski JM, Lebiedzinska M, Karkucinska-Wieckowska A, Wojtala A, Pronicki M, Duszynski J, Pinton P et al (2013) Cardiac mitochondrial dysfunction during hyperglycemia—the role of oxidative stress and p66Shc signaling. Int J Biochem Cell Biol 45(1):114–122
Rolo AP, Palmeira CM (2006) Diabetes and mitochondrial function: role of hyperglycemia and oxidative stress. Toxicol Appl Pharmacol 212(2):167–178
Palmeira CM, Rolo AP, Berthiaume J, Bjork JA, Wallace KB (2007) Hyperglycemia decreases mitochondrial function: the regulatory role of mitochondrial biogenesis. Toxicol Appl Pharmacol 225(2):214–220
Amaral S, Oliveira PJ, Ramalho-Santos J (2008) Diabetes and the impairment of reproductive function: possible role of mitochondria and reactive oxygen species. Curr Diabetes Rev 4(1):46–54
Wang J, Li G, Wang Z, Zhang X, Yao L, Wang F, Liu S, Yin J et al (2012) High glucose-induced expression of inflammatory cytokines and reactive oxygen species in cultured astrocytes. Neuroscience 202:58–68
van Dam PS, van Asbeck BS, Bravenboer B, van Oirschot JF, Gispen WH, Marx JJ (1998) Nerve function and oxidative stress in diabetic and vitamin E-deficient rats. Free Radic Biol Med 24(1):18–26
Singh JN, Jain G, Sharma SS (2013) In vitro hyperglycemia enhances sodium currents in dorsal root ganglion neurons: an effect attenuated by carbamazepine. Neuroscience 232:64–73
Zou Y, Zhao T, Mao G, Zhang M, Zheng D, Feng W, Wang W, Wu X et al (2014) Isolation, purification and characterisation of selenium-containing polysaccharides and proteins in selenium-enriched Radix puerariae. J Sci Food Agric 94(2):349–358
Fan C, Zheng W, Fu X, Li X, Wong YS, Chen T (2014) Strategy to enhance the therapeutic effect of doxorubicin in human hepatocellular carcinoma by selenocystine, a synergistic agent that regulates the ROS-mediated signaling. Oncotarget 5(9):2853–2863
Fan C, Zheng W, Fu X, Li X, Wong YS, Chen T (2014) Enhancement of auranofin-induced lung cancer cell apoptosis by selenocystine, a natural inhibitor of TrxR1 in vitro and in vivo. Cell Death Dis 5:e1191
Fan C, Chen J, Wang Y, Wong YS, Zhang Y, Zheng W, Cao W, Chen T (2013) Selenocystine potentiates cancer cell apoptosis induced by 5-fluorouracil by triggering reactive oxygen species-mediated DNA damage and inactivation of the ERK pathway. Free Radic Biol Med 65:305–316
Pillai R, Uyehara-Lock JH, Bellinger FP (2014) Selenium and selenoprotein function in brain disorders. IUBMB Life 66(4):229–239
Ozbal S, Erbil G, Kocdor H, Tugyan K, Pekcetin C, Ozogul C (2008) The effects of selenium against cerebral ischemia-reperfusion injury in rats. Neurosci Lett 438(3):265–269
Bellinger FP, Raman AV, Reeves MA, Berry MJ (2009) Regulation and function of selenoproteins in human disease. Biochem J 422(1):11–22
Kunwar A, Bag PP, Chattopadhyay S, Jain VK, Priyadarsini KI (2011) Anti-apoptotic, anti-inflammatory, and immunomodulatory activities of 3,3′-diselenodipropionic acid in mice exposed to whole body gamma-radiation. Arch Toxicol 85(11):1395–1405
Kunwar A, Bansal P, Kumar SJ, Bag PP, Paul P, Reddy ND, Kumbhare LB, Jain VK et al (2010) In vivo radioprotection studies of 3,3′-diselenodipropionic acid, a selenocystine derivative. Free Radic Biol Med 48(3):399–410
Cao W, Li X, Zheng S, Zheng W, Wong YS, Chen T (2014) Selenocysteine derivative overcomes TRAIL resistance in melanoma cells: evidence for ROS-dependent synergism and signaling crosstalk. Oncotarget 5(17):7431–7445
Yagami T, Yamamoto Y, Koma H (2014) The role of secretory phospholipase A(2) in the central nervous system and neurological diseases. Mol Neurobiol 49(2):863–876
Cho SW, Park JS, Heo HJ, Park SW, Song S, Kim I, Han YM, Yamashita JK et al (2014) Dual modulation of the mitochondrial permeability transition pore and redox signaling synergistically promotes cardiomyocyte differentiation from pluripotent stem cells. J Am Heart Assoc 3(2):e000693
Bove J, Martinez-Vicente M, Dehay B, Perier C, Recasens A, Bombrun A, Antonsson B, Vila M (2014) BAX channel activity mediates lysosomal disruption linked to Parkinson disease. Autophagy 10(5):889–900
Gomez-Crisostomo NP, Lopez-Marure R, Zapata E, Zazueta C, Martinez-Abundis E (2013) Bax induces cytochrome c release by multiple mechanisms in mitochondria from MCF7 cells. J Bioenerg Biomembr 45(5):441–448
Simonishvili S, Jain MR, Li H, Levison SW, Wood TL (2013) Identification of Bax-interacting proteins in oligodendrocyte progenitors during glutamate excitotoxicity and perinatal hypoxia-ischemia. ASN Neuro 5(5):e00131
Liu MH, Yuan C, He J, Tan TP, Wu SJ, Fu HY, Liu J, Yu S et al (2014) Resveratrol protects PC12 cells from high glucose-induced neurotoxicity via PI3K/Akt/FoxO3a pathway. Cell Mol Neurobiol 35:513–522
Chen T, Wong YS (2008) Selenocystine induces apoptosis of A375 human melanoma cells by activating ROS-mediated mitochondrial pathway and p53 phosphorylation. Cell Mol Life Sci 65(17):2763–2775
Park JH, Lee SW, Yang SW, Yoo HM, Park JM, Seong MW, Ka SH, Oh KH et al (2014) Modification of DBC1 by SUMO2/3 is crucial for p53-mediated apoptosis in response to DNA damage. Nat Commun 5:5483
Coulpier M, Anders J, Ibanez CF (2002) Coordinated activation of autophosphorylation sites in the RET receptor tyrosine kinase: importance of tyrosine 1062 for GDNF mediated neuronal differentiation and survival. J Biol Chem 277(3):1991–1999
Pitts MW, Byrns CN, Ogawa-Wong AN, Kremer P, Berry MJ (2014) Selenoproteins in nervous system development and function. Biol Trace Elem Res 161(3):231–245
Bellinger FP, He QP, Bellinger MT, Lin Y, Raman AV, White LR, Berry MJ (2008) Association of selenoprotein p with Alzheimer’s pathology in human cortex. J Alzheimers Dis 15(3):465–472
Shahar A, Patel KV, Semba RD, Bandinelli S, Shahar DR, Ferrucci L, Guralnik JM (2010) Plasma selenium is positively related to performance in neurological tasks assessing coordination and motor speed. Mov Disord 25(12):1909–1915
Mousavi SH, Tayarani NZ, Parsaee H (2010) Protective effect of saffron extract and crocin on reactive oxygen species-mediated high glucose-induced toxicity in PC12 cells. Cell Mol Neurobiol 30(2):185–191
Eslami H, Sharifi AM, Rahimi H, Rahati M (2014) Protective effect of telmisartan against oxidative damage induced by high glucose in neuronal PC12 cell. Neurosci Lett 558:31–36
Yuan J, Huang G, Xiao Z, Lin L, Han T (2013) Overexpression of beta-NGF promotes differentiation of bone marrow mesenchymal stem cells into neurons through regulation of AKT and MAPK pathway. Mol Cell Biochem 383(1-2):201–211
Wu J, Jiang H, Luo S, Zhang M, Zhang Y, Sun F, Huang S, Li H (2013) Caspase-mediated cleavage of C53/LZAP protein causes abnormal microtubule bundling and rupture of the nuclear envelope. Cell Res 23(5):691–704
Xing S, Zhang J, Dang C, Liu G, Zhang Y, Li J, Fan Y, Pei Z et al (2014) Cerebrolysin reduces amyloid-beta deposits, apoptosis and autophagy in the thalamus and improves functional recovery after cortical infarction. J Neurol Sci 337(1-2):104–111
Vandegriff KD, Malavalli A, Lohman J, Young MA, Terraneo L, Virgili E, Bianciardi P, Caretti A et al (2014) Impact of acellular hemoglobin-based oxygen carriers on brain apoptosis in rats. Transfusion 54(8):2045–2054
Wu Q, Tang ZH, Peng J, Liao L, Pan LH, Wu CY, Jiang ZS, Wang GX et al (2014) The dual behavior of PCSK9 in the regulation of apoptosis is crucial in Alzheimer’s disease progression (Review). Biomed Rep 2(2):167–171
Kim SY, Shim MS, Kim KY, Weinreb RN, Wheeler LA, Ju WK (2014) Inhibition of cyclophilin D by cyclosporin A promotes retinal ganglion cell survival by preventing mitochondrial alteration in ischemic injury. Cell Death Dis 5:e1105
Hou Y, Ghosh P, Wan R, Ouyang X, Cheng H, Mattson MP, Cheng A (2014) Permeability transition pore-mediated mitochondrial superoxide flashes mediate an early inhibitory effect of amyloid beta1-42 on neural progenitor cell proliferation. Neurobiol Aging 35(5):975–989
Li DW, Yao M, Dong YH, Tang MN, Chen W, Li GR, Sun BQ (2014) Guanosine exerts neuroprotective effects by reversing mitochondrial dysfunction in a cellular model of Parkinson’s disease. Int J Mol Med 34(5):1358–1364
Li D, Liu M, Tao TQ, Song DD, Liu XH, Shi DZ (2014) Panax quinquefolium saponin attenuates cardiomyocyte apoptosis and opening of the mitochondrial permeability transition pore in a rat model of ischemia/reperfusion. Cell Physiol Biochem 34(4):1413–1426
Gu W, Hou X, Zhou H, Li C (2014) Protective effect of shen-fu injection on neuronal mitochondrial function in a porcine model of prolonged cardiac arrest. Evid Based Complement Alternat Med 2014:523847
Li J, Yu W, Li XT, Qi SH, Li B (2014) The effects of propofol on mitochondrial dysfunction following focal cerebral ischemia-reperfusion in rats. Neuropharmacology 77:358–368
Ge R, Ma WH, Li YL, Li QS (2013) Apoptosis induced neurotoxicity of Di-n-butyl-di-(4-chlorobenzohydroxamato) Tin (IV) via mitochondria-mediated pathway in PC12 cells. Toxicol In Vitro 27(1):92–102
Jung SY, Kim DY, Yune TY, Shin DH, Baek SB, Kim CJ (2014) Treadmill exercise reduces spinal cord injury-induced apoptosis by activating the PI3K/Akt pathway in rats. Exp Ther Med 7(3):587–593
Goncalves AC, Barbosa-Ribeiro A, Alves V, Silva T, Sarmento-Ribeiro AB (2013) Selenium compounds induced ROS-dependent apoptosis in myelodysplasia cells. Biol Trace Elem Res 154(3):440–447
Wang Y, Wu Y, Luo K, Liu Y, Zhou M, Yan S, Shi H, Cai Y (2013) The protective effects of selenium on cadmium-induced oxidative stress and apoptosis via mitochondria pathway in mice kidney. Food Chem Toxicol 58:61–67
Sun H, Leng T, Zeng Z, Gao X, Inoue K, Xiong ZG (2013) Role of TRPM7 channels in hyperglycemia-mediated injury of vascular endothelial cells. PLoS One 8(11):e79540
van Veen S, Sorensen DM, Holemans T, Holen HW, Palmgren MG, Vangheluwe P Cellular function and pathological role of ATP13A2 and related P-type transport ATPases in Parkinson’s disease and other neurological disorders. Front Mol Neurosci 7:48
Gaspar JM, Martins A, Cruz R, Rodrigues CM, Ambrosio AF, Santiago AR (2013) Tauroursodeoxycholic acid protects retinal neural cells from cell death induced by prolonged exposure to elevated glucose. Neuroscience 253:380–388
Odinokova IV, Sung KF, Mareninova OA, Hermann K, Evtodienko Y, Andreyev A, Gukovsky I, Gukovskaya AS (2009) Mechanisms regulating cytochrome c release in pancreatic mitochondria. Gut 58(3):431–442
Renaud J, Bournival J, Zottig X, Martinoli MG (2014) Resveratrol protects DAergic PC12 cells from high glucose-induced oxidative stress and apoptosis: effect on p53 and GRP75 localization. Neurotox Res 25(1):110–123
Guan L, Huang F, Li Z, Han B, Jiang Q, Ren Y, Yang Y, Xu C (2008) P53 transcription-independent activity mediates selenite-induced acute promyelocytic leukemia NB4 cell apoptosis. BMB Rep 41(10):745–750
Aminzadeh A, Dehpour AR, Safa M, Mirzamohammadi S, Sharifi AM (2014) Investigating the protective effect of lithium against high glucose-induced neurotoxicity in PC12 cells: involvements of ROS, JNK and P38 MAPKs, and apoptotic mitochondria pathway. Cell Mol Neurobiol 34(8):1143–1150
Li Q, Guo HC, Maslov LN, Qiao XW, Zhou JJ, Zhang Y (2014) Mitochondrial permeability transition pore plays a role in the cardioprotection of CB2 receptor against ischemia-reperfusion injury. Can J Physiol Pharmacol 92(3):205–214
Acknowledgments
The study was supported by the National Natural Science Foundation of China no. 81471212, 81271275, 81070947, and 30770759 to B.-L. Sun and no. 81301018 to Z.-Y Zhang; and Natural Science Foundation of Shandong no. ZR2012HZ006 to B.-L. Sun, no. ZR2015HQ009 to C.-D. Fan, and no. ZR2015PH003 to X.-Y. Fu.
Conflict of Interest
The authors declare that they have no competing interests.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Kun Wang and Xiao-yan Fu contributed equally to this work.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 584 kb)
Rights and permissions
About this article
Cite this article
Wang, K., Fu, Xy., Fu, Xt. et al. DSePA Antagonizes High Glucose-Induced Neurotoxicity: Evidences for DNA Damage-Mediated p53 Phosphorylation and MAPKs and AKT Pathways. Mol Neurobiol 53, 4363–4374 (2016). https://doi.org/10.1007/s12035-015-9373-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-015-9373-1