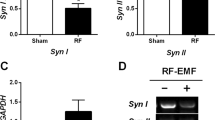
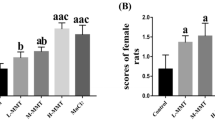
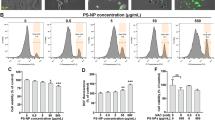
Abstract
Parkinson’s disease (PD) is a neurodegenerative disorder characterized by dopaminergic neuron loss, with an etiopathogenesis involving both genetic and environmental factors. The occupational/residential exposure to the electromagnetic fields has been recently associated with an increased risk of neurodegenerative diseases; it has been thus proposed that the extremely low frequency magnetic field (ELF-MF) may contribute to neurodegenerative etiopathogenesis, as its interaction with biological systems directly impairs redox homeostasis in specific areas of the brain. The molecular mechanisms elicited by ELF-MF, and their potential involvement in PD onset, still remain unclear. To this end, we set up a generator of ELF-MF able to stably and homogeneously reproduce environmental prolonged exposure to ELF-MF (50 Hz, 1 mT). Results obtained indicate that ELF-MF exposure alters cell response of SH-SY5Y cells to MPP+. We demonstrate that ELF-MF does not affect per se survival, shape, and morphology of both proliferating and differentiated SH-SY5Y cells but significantly impairs redox homeostasis and thiol content, triggering an increase in protein carbonylation. As a result, toxicity of MPP+, even at low doses, is highly enhanced in ELF-MF-exposed cells due to a significant increase in ROS levels, potentiation of oxidative damage, and induction of a caspase-dependent apoptosis. Pre-incubation with the thiol antioxidants N-acetyl-l-cysteine and GSH ethyl-ester significantly reduces the extent of oxidative damage and protects cells from death induced by the combined treatment ELF-MF/MPP+. Taken overall, our results demonstrate the redox-based molecular interaction between ELF-MF and PD neurotoxins in vitro, and open a new scenario for defining the synergy of environmental factors in PD onset.







Similar content being viewed by others
Abbreviations
- AD:
-
Alzheimer’s disease
- ALS:
-
Amyotrophic lateral sclerosis
- B field:
-
Induction magnetic field
- BSO:
-
Buthionine-S,R-sulfoximine
- CMFDA:
-
5-chloromethylfluorescein diacetate
- DAergic:
-
Dopaminergic
- DHE:
-
Dihydroethidium
- E field:
-
Electric field
- ELF-MF:
-
Extremely low frequency magnetic fields
- GSH:
-
Reduced glutathione
- GSHest:
-
Reduced glutathione ethyl ester
- H2-DCFDA:
-
2′,7′-Dichlorofluorescin diacetate
- LBs:
-
Lewy bodies
- MFI:
-
Mean fluorescence intensity
- MPP+ :
-
1-methyl-4-phenylpyridinium
- MPTP:
-
Methyl-4-phenyl-1,2,3,6-tetra-hydropyridine
- NAC:
-
N-Acetyl-l-cysteine
- PD:
-
Parkinson’s disease
- PI:
-
Propidium iodide
- PMA:
-
Phorbol 12-myristate 13-acetate
- RA:
-
Retinoic acid
- RMS:
-
Root-mean-square
- ROS:
-
Reactive oxygen species
- SN:
-
Substrantia nigra
References
Dauer W, Przedborski S (2003) Parkinson’s disease: mechanisms and models. Neuron 39:889–909, Review
Thomas B, Beal MF (2007) Parkinson’s disease. Hum Mol Genet 16:R183–R194
Binukumar BK, Bal A, Kandimalla RJ, Gill KD (2010) Nigrostriatal neuronal death following chronic dichlorvos exposure: crosstalk between mitochondrial impairments, α synuclein aggregation, oxidative damage and behavioral changes. Mol Brain 3:35
Evans PH (1993) Free radicals in brain metabolism and pathology. Br Med Bull 49(3):577–587, Review
Alam ZI, Jenner A, Daniel SE, Lees AJ, Cairns N, Marsden CD, Jenner P, Halliwell B (1997) Oxidative DNA damage in the parkinsonian brain: an apparent selective increase in 8-hydroxyguanine levels in substantia nigra. J Neurochem 69(3):1196–1203
Schapira AHV (2007) Mitochondrial dysfunction in Parkinson’s disease. Cell Death Differ 14(7):1261–1266
Smeyne M, Smeyne RJ (2013) Glutathione metabolism and Parkinson’s disease. Free Radic Biol Med 62:13–25, Review
Piccini P, Burn DJ, Ceravolo R, Maraganore D, Brooks DJ (1999) The role of inheritance in sporadic Parkinson’s disease: evidence from a longitudinal study of dopaminergic function in twins. Ann Neurol 45(5):577–582
Elbaz A, Dufouil C, Alpérovitch A (2007) Interaction between genes and environment in neurodegenerative diseases. C R Biol 330(4):318–328, Review
Kieburtz K, Wunderle KB (2013) Parkinson’s disease: evidence for environmental risk factors. Mov Disord 28(1):8–13, Review
Gorell JM, Johnson CC, Rybicki BA, Peterson EL, Richardson RJ (1998) The risk of Parkinson’s disease with exposure to pesticides, farming, well water, and rural living. Neurology 50(5):1346–1350
Savitz DA, Checkoway H, Loomis DP (1998) Magnetic field exposure and neurodegenerative disease mortality among electric utility workers. Epidemiology 4:398–404
Davanipour Z, Tseng CC, Lee PJ, Sobel E (2007) A case-control study of occupational magnetic field exposure and Alzheimer’s disease: results from the California Alzheimer’s Disease Diagnosis and Treatment Centers. BMC Neurol 7:13
Li CY, Sung FC (2003) Association between occupational exposure to power frequency electromagnetic fields and amyotrophic lateral sclerosis: a review. Am J Ind Med 43(2):212–220
Consales C, Merla C, Marino C, Benassi B (2012) Electromagnetic fields, oxidative stress, and neurodegeneration. Int J Cell Biol 2012:683897
Wechsler LS, Checkoway H, Franklin GM, Costa LG (1991) A pilot study of occupational and environmental risk factors for Parkinson’s disease. Neurotoxicology 12(3):387–392
Adair RK (1999) Effects of very weak magnetic fields on radical pair reformation. Bioelectromagnetics 20(4):255–263
Samano JM, Torres-Duran PV, Juarez-Oropeza MA, Verdugo-Diaz L (2012) Effects of acute extremely low frequency electromagnetic field exposure on the antioxidant status and lipid levels in rat brain. Arch Med Res 43:183–189
Di Loreto S, Falone S, Caracciolo V, Sebastiani P, D’Alessandro A, Mirabilio A, Zimmitti V, Amicarelli F (2009) Fifty hertz extremely low-frequency magnetic field exposure elicits redox and trophic response in rat-cortical neurons. J Cell Physiol 219(2):334–343
Morabito C, Guarnieri S, Fanò G, Mariggiò MA (2010) Effects of acute and chronic low frequency electromagnetic field exposure on PC12 cells during neuronal differentiation. Cell Physiol Biochem 26(6):947–958
Falone S, Grossi MR, Cinque B, D’Angelo B, Tettamanti E et al (2007) Fifty Hertz extremely low frequency electromagnetic field causes changes in redox and differentiative status in neuroblastoma cells. Int J Biochem Cell Biol 39(11):2093–2106
Beal MF (2001) Experimental models of Parkinson’s disease. Nat Rev Neurosci 2(5):325–334, Review
Langston JW, Ballard PA Jr (1983) Parkinson’s disease in a chemist working with 1-methyl-4-phenyl-1,2,5,6-tetrahydropyridine. N Engl J Med 309(5):310
Burns RS, Chiueh CC, Markey SP, Ebert MH, Jacobowitz DM, Kopin IJ (1983) A primate model of parkinsonism: selective destruction of dopaminergic neurons in the pars compacta of the substantia nigra by N-methyl-4-phenyl-1,2,3,6-tetrahydropyridine. Proc Natl Acad Sci U S A 80(14):4546–4550
Suzuki K, Mizuno Y, Yoshida M (1990) Effects of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-like compounds on mitochondrial respiration. Adv Neurol 53:215–218
Presgraves SP, Ahmed T, Borwege S, Joyce JN (2004) Terminally differentiated SH-SY5Y cells provide a model system for studying neuroprotective effects of dopamine agonists. Neurotox Res 5(8):579–598
Lodato R, Merla C, Pinto R, Mancini S, Lopresto V, Lovisolo GA (2013) Complex magnetic field exposure system for in vitro experiments at intermediate frequencies. Bioelectromagnetics 34(3):211–219
Schuderer J, Oesch W, Felber N, Spät D, Kuster N (2004) In vitro exposure apparatus for ELF magnetic fields. Bioelectromagnetics 25(8):582–591
Kuster N, Schuderer J, Christ A, Futter P, Ebert S (2004) Guidance for exposure design of human studies addressing health risk evaluations of mobile phones. Bioelectromagnetics 25(7):524–529
Cardaci S, Filomeni G, Rotilio G, Ciriolo MR (2010) p38MAPK/p53 signaling axis mediates neuronal apoptosis in response to tetrahydrobiopterin-induced oxidative stress and glucose uptake inhibition: implication for neurodegeneration. Biochem J 430:439–451
Filomeni G, Graziani I, De Zio D, Dini L, Centonze D, Rotilio G, Ciriolo MR (2012) Neuroprotection of kaempferol by autophagy in models of rotenone-mediated acute toxicity: possible implications for Parkinson’s disease. Neurobiol Aging 33(4):767–785
Poot M, Kavanagh TJ, Kang HC, Haugland RP, Rabinovitch PS (1991) Flow cytometric analysis of cell cycle-dependent changes in cell thiol level by combining a new laser dye with Hoechst 33342. Cytometry 12(2):184–187
Darzynkiewicz Z, Juan G, Li X, Gorczyca W, Murakami T, Traganos F (1997) Cytometry in cell necrobiology: analysis of apoptosis and accidental cell death (necrosis). Cytometry 27(1):1–20
Wilkins RC, Kutzner BC, Truong M, Sanchez-Dardon J, McLean JR (2002) Analysis of radiation-induced apoptosis in human lymphocytes: flow cytometry using Annexin V and propidium iodide versus the neutral comet assay. Cytometry 48(1):14–19
Metkar S, Wang B, Catalan E, Anderluh G, Gilbert RJ, Pardo J, Froelich CJ (2011) Perforin rapidly induces plasma membrane phospholipid flip-flop. PLoS One 6(9), e24286
Wong CM, Cheema AK, Zhang L, Suzuki YJ (2008) Protein carbonylation as novel mechanism in redox signaling. Circ Res 102:310–318
Nicotra A, Parvez S (2002) Apoptotic molecules and MPTP-induced cell death. Neurotoxicol Teratol 24(5):599–605
Xie HR, Hu LS, Li GY (2010) SH-SY5Y human neuroblastoma cell line: in vitro cell model of dopaminergic neurons in Parkinson’s disease. Chin Med J (Engl) 123(8):1086–1092, Review
Korecka JA, van Kesteren RE, Blaas E, Spitzer SO, Kamstra JH, Smit AB, Swaab DF, Verhaagen J et al (2003) Phenotypic characterization of retinoic acid differentiated SH-SY5Y cells by transcriptional profiling. PLoS One 8(5), e63862
Wertheimer N, Leeper E (1979) Electrical wiring configurations and childhood cancer. Am J Epidemiol 109(3):273–284
Loomis DP, Savitz DA (1990) Mortality from brain cancer and leukaemia among electrical workers. Br J Ind Med 47(9):633–638
Mattsson MO, Simkò (2012) Is there a relationship between extremely low frequency magnetic field exposure, inflammation and neurodegenerative diseases? A review of in vivo and in vitro experimental evidences. Toxicology 301:1–12
Di Lazzaro V, Capone F, Apollonio F, Borea PA, Cadossi R, Fassina L, Grassi C, Liberti M et al (2013) A consensus panel review of central nervous system effects of the exposure to low-intensity extremely low frequency magnetic fields. Brain Stimul 6:469–476
Gordon J, Amini S, White MK (2013) General overview of neuronal cell culture. Methods Mol Biol 1078:1–8
International Commission on Non-Ionizing Radiation Protection (2010) Guidelines for limiting exposure to time-varying electric and magnetic fields (1 Hz to 100 kHz). Health Phys 99(6):818–836
Reale M, Kamal MA, Patruno A, Costantini E, D’Angelo C, Pesce M, Greig NH (2014) Neuronal cellular responses to extremely low frequency electromagnetic field exposure: implications regarding oxidative stress and neurodegeneration. PLoS One 9(8), e104973
Luukkonen J, Liimatainen A, Juutilainen J, Naarala J (2014) Induction of genomic instability, oxidative processes, and mitochondrial activity by 50Hz magnetic fields in human SH-SY5Y neuroblastoma cells. Mutat Res Fundam Mol Mech Mutagen 760:33–41
Cadet JL, Brannock C (1998) Free radicals and the pathobiology of brain dopamine systems. Neurochem Int 32:117–131
Infanger DW, Sharma RV, Davisson RL (2006) NADPH oxidases of the brain: distribution, regulation, and function. Antioxid Redox Signal 8(9-10):1583–1596, Review
Pearce RK, Owen A, Daniel S, Jenner P, Marsden CD (1997) Alterations in the distribution of glutathione in the substantia nigra in Parkinson’s disease. J Neural Transm 104(6-7):661–677
Jellinger KA (2008) A critical reappraisal of current staging of Lewy-related pathology in human brain. Acta Neuropathol 116:1–16
Chinta SJ, Andersen JK (2006) Reversible inhibition of mitochondrial complex I activity following chronic dopaminergic glutathione depletion in vitro: implications for Parkinson’s disease. Free Radic Biol Med 41:1442–1448
Holmay MJ, Terpstra M, Coles L, Mishra U, Ahlskog M, Oz G, Cloyd JC, Tuite PJ (2013) N-acetylcysteine boosts brain and blood glutathione in Gaucher and Parkinson disease. Clin Neuropharmacol 36(4):103–106
Berman AE, Chan WY, Brennan AM et al (2011) N-acetylcysteine prevents loss of dopaminergic neurons in the EAAC1-/- mouse. Ann Neurol 69:509–520
Wüllner U, Löschmann PA, Schulz JB, Schmid A, Dringen R, Eblen F, Turski L, Klockgether T (1996) Glutathione depletion potentiates MPTP and MPP+ toxicity in nigral dopaminergic neurones. Neuroreport 7(4):921–923
Bizzozero OA, Ziegler JL, De Jesus G, Bolognani F (2006) Acute depletion of reduced glutathione causes extensive carbonylation of rat brain proteins. J Neurosci Res 83(4):656–667
Chinta SJ, Kumar MJ, Hsu M et al (2007) Inducible alterations of glutathione levels in adult dopaminergic midbrain neurons result in nigrostriatal degeneration. J Neurosci 27:13997–14006
Ma Q, Deng P, Zhu G, Liu C, Zhang L, Zhou Z, Luo X, Li M et al (2014) Extremely low frequency electromagnetic fields affect transcript levels of neuronal differentiation-related genes in embryonic neural stem cells. PLoS One 9(3), e90041
Manikonda PK, Rajendrap, Devendranath D, Gunasekaran B, Channakeshava, Aradhya RS, Sashidar RB, Subramanyam C (2007) Influence of the extremely low frequency magnetic field and Ca2+ signaling and NMDA receptor in rat hippocampus. Neurosci Lett 413(2):145–149
Acknowledgments
G.F. is supported by grants from the National Ministry of Health, Young Italian Researcher Grant-2008 (Grant GR-2008-1138121), and from the Italian Association for Cancer Research, AIRC-MFAG 2011 (Grant 11452). We are very grateful to Francesca Pacchierotti for her helpful criticisms and scientific support and to Martin W. Bennett for manuscript editing.
Conflict of Interest
The authors declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
Online Resource 1
(PPT 359 kb)
Online Resource 2
(PPT 180 kb)
Rights and permissions
About this article
Cite this article
Benassi, B., Filomeni, G., Montagna, C. et al. Extremely Low Frequency Magnetic Field (ELF-MF) Exposure Sensitizes SH-SY5Y Cells to the Pro-Parkinson’s Disease Toxin MPP+ . Mol Neurobiol 53, 4247–4260 (2016). https://doi.org/10.1007/s12035-015-9354-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-015-9354-4