Abstract
Parkinson’s disease (PD) is pathologically characterized by selective loss of dopaminergic neurons in the midbrain and the existence of intracellular protein inclusions termed Lewy bodies, largely composed of α-synuclein. Genetic studies have revealed that rare point mutations in the gene encoding α-synuclein including A30P, A53T, and E46K are associated with familial forms of PD, indicating a pathological role for mutant α-synuclein in PD etiology. However, the mechanisms underlying the neuronal toxicity of mutant α-synuclein are still to be elucidated. Growing evidence has suggested a deleterious effect of mutant α-synuclein on the autophagy-lysosome pathway. In this study, we discovered that overexpression of human E46K mutant α-synuclein impaired macroautophagy in mammalian cells. Our data showed that overexpression of E46K mutant α-synuclein impaired autophagy at an early stage of autophagosome formation via the c-Jun N-terminal kinase 1 (JNK1)-Bcl-2 but not the mammalian target of rapamycin (mTOR) pathway. Overexpressed E46K mutant α-synuclein inhibited JNK1 activation, leading to a reduced Bcl-2 phosphorylation and increased association between Bcl-2 and Beclin1, further disrupting the formation of Beclin1/hVps34 complex, which is essential for autophagy initiation. Furthermore, overexpression of E46K mutant α-synuclein increased the vulnerability of differentiated PC12 cells to rotenone treatment, which would be partly due to its inhibitory effects on autophagy. Our findings may shed light on the potential roles of mutant α-synuclein in the pathogenesis of PD.








Similar content being viewed by others
References
Lees AJ, Hardy J, Revesz T (2009) Parkinson’s disease. Lancet 373(9680):2055–2066
Bartels AL, Leenders KL (2009) Parkinson’s disease: the syndrome, the pathogenesis and pathophysiology. Cortex 45(8):915–921
Banerjee R, Beal MF, Thomas B (2010) Autophagy in neurodegenerative disorders: pathogenic roles and therapeutic implications. Trends Neurosci 33(12):541–549
Nixon RA (2006) Autophagy in neurodegenerative disease: friend, foe or turncoat? Trends Neurosci 29(9):528–535
Martinez-Vicente M, Cuervo AM (2007) Autophagy and neurodegeneration: when then cleaning crew goes on strike. Lancet Neurol 6(4):352–361
Pan T, Kondo S, Le W, Jankovic J (2008) The role of autophagy-lysosome pathway in neurodegeneration associated with Parkinson’s disease. Brain 131:1969–1978
Rubinsztein DC (2006) The roles of intracellular protein-degradation pathways in neurodegeneration. Nature 443(7113):780–786
Xilouri M, Stefanis L (2011) Autophagic pathways in Parkinson disease and related disorders. Expert Rev Mol Med 13:e8
Klionsky DJ, Emr SD (2000) Autophagy as a regulated pathway of cellular degradation. Science 290(5497):1717–1721
Ravikumar B, Sarkar S, Davies J et al (2010) Regulation of mammalian autophagy in physiology and pathophysiology. Physiol Rev 90(4):1383–1435
Shintani T, Klisonsky DJ (2004) Autophagy in health and disease: a double-edged sword. Science 306(5698):990–995
Cuervo AM (2004) Autophagy: in sickness and in health. Trends Cell Biol 14(2):70–77
Kroemer G, Marino G, Levine B (2010) Autophagy and the integrated stress response. Mol Cell 40(2):280–293
Levine B, Kroemer G (2008) Autophagy in the pathogenesis of disease. Cell 132(1):27–42
Cuervo AM (2010) Chaperone-mediated autophagy: selectivity pays off. Trends Endocrinol Metab 21(3):142–150
Yang Z, Klionsky DJ (2010) Eaten alive: a history of macroautophagy. Nat Cell Biol 12(9):814–822
Kundu M, Thompson CB (2008) Autophagy: basic principles and relevance to disease. Annu Rev Pathol 3:427–455
Mizushima N (2007) Autophagy: process and function. Genes Dev 21(22):2861–2873
Levine B (2005) Eating oneself and uninvited guests: autophagy-related pathways in cellular defense. Cell 120(2):159–162
Laplante M, Sabatini DM (2012) mTOR signaling in growth control and disease. Cell 149(2):274–293
Sengupta S, Peterson T, Sabatini DM (2010) Regulation of the mTOR complex 1 pathway by nutrients, growth factors, and stress. Mol Cell 40(2):310–322
He C, Klisonsky DJ (2009) Regulation mechanisms and signaling pathways of autophagy. Annu Rev Genet 43:67–93
Pattingre S, Tassa A, Qu X, Garuti R, Liang XH, Mizushima N, Packer M, Schneider MD, Levine B (2005) Bcl-2 antiapoptotic proteins inhibit Beclin1-dependent autophagy. Cell 122(6):927–939
Pattingre S, Espert L, Biard-Piechaczyk M, Codogno P (2008) Regulation of macroautophagy by mTOR and Beclin1 complex. Biochimie 90(2):313–323
Wei Y, Pattingre S, Sinha S, Bassik M, Levine B (2008) JNK1-mediated phosphorylation of Bcl-2 regulates starvation-induced autophagy. Mol Cell 30(6):678–688
Polymeropoulos MH, Lavedan C, Leroy E et al (1997) Mutation in the α-synuclein gene identified in families with Parkinson’s disease. Science 276(5321):2045–2047
Kruger R, Kuhn W, Muller T et al (1998) Ala30Pro mutation in the gene encoding α-synuclein in Parkinson’s disease. Nat Genet 18:106–108
Zarranz JJ, Alegre J, Gómez-Esteban JC et al (2004) The new mutation, E46K, of α-synuclein causes Parkinson and Lewy Body dementia. Ann Neurol 55(2):164–173
Cuvreo AM, Stefanis L, Fredenburg R, Lansbury PT, Sulzer D (2004) Impaired degradation of mutant alpha-synuclein by chaperone-mediated autophagy. Science 305(5688):1292–1295
Stefanis L, Larsen KE, Rideout HJ, Sulzer D, Greene LA (2001) Expression of A53T mutant but not wild-type alpha-synuclein in PC12 cells induces alterations of the ubiquitin-dependent degradation systems, loss of dopamine release, and autophagic cell death. J Neurosci 21(24):9549–9560
Martinez-Vicente M, Talloczy Z, Kaushik S, Massey AC, Mazzulli J, Mosharov EV, Hodara R, Fredenburg R, Wu DC, Follenzi A, Dauer W, Przedborski S, Ischiropoulos H, Lansbury PT, Sulzer D, Cuervo AM (2008) Dopamine-modified alpha-synuclein blocks chaperone-mediated autophagy. J Clin Invest 118(2):777–788
Yuan YH, Jin J, Yang B, Zhang W, Hu JF, Chen NH (2008) Overexpressed alpha-synuclein regulated the nuclear factor-kappaB signal pathway. Cell Mol Neurobiol 28(1):21–33
Ma KL, Song LK, Yuan YH, Zhang Y, Han N, Gao K, Chen NH (2013) The nuclear accumulation of alpha-synuclein is mediated by importin alpha and promotes neurotoxicity by accelerating the cell cycle. Neuropharmacology. doi:10.1016/j.neuropharm.2013.07.035
Korolchuk VI, Mansilla A, Menzies FM, Rubinsztein DC (2009) Autophagy inhibition compromises degradation of ubiquitin-proteasome pathway substrates. Mol Cell 33(4):517–527
Sarkar S, Korolchuk VI, Renna M, Imarisio M, Fleming A, Williams A, Garcia-Arencibia M, Rose C, Luo S, Underwood BR, Kroemer G (2011) Complex inhibitory effects of nitric oxide on autophagy. Mol Cell 43(1):19–32
Martinez-Vicente M, Talloczy Z, Wong E, Tang G, Koga H, Kaushik S, de Vries R, Arias E, Harris S, Sulzer D, Cuervo AM (2010) Cargo recognition failure is responsible for inefficient autophagy in Huntington’s disease. Nat Neurosci 13(5):567–576
Pattingre S, Bauvy C, Carpentier S, Levade T, Levine B (2009) Role of JNKl-dependent Bcl-2 phosphorylation in ceramide-induced macroautophagy. J Biol Chem 284(5):2719–2728
Shvets E, Fass E, Elazar Z (2008) Utilizing flow cytometry to monitor autophagy in living mammalian cells. Autophagy 4(5):621–628
Song XY, Hu JF, Chu SF, Li ZP, Wu DH, Ji HJ, Yuan YH, Zhu ZX, Han N, Liu G, Chen NH (2013) IMM-H004, a novel coumarin derivative compound, protects against amyloid beta-induced neurotoxicity through a mitochondrial-dependent pathway. Neuroscience 242:28–38
Kirkin V, McEwan DG, Novak I, Dikic I (2009) A role for ubiquitin in selective autophagy. Mol Cell 34(3):259–269
Lamark T, Kirkin V, Dikic I, Johansen T (2009) NBR1 and p62 as cargo receptors for selective autophagy of ubiquitinated targets. Cell Cycle 8(13):1986–1990
Klionsky DJ, Abdalla FC, Abeliovich H et al (2012) Guidelines for the use and interpretation of assays for monitoring autophagy. Autophagy 8(4):445–544
Mizushima N, Levine B, Cuervo AM, Klionsky DJ (2008) Autophagy fight disease through cellular self-digestion. Nature 451(7182):1069–1075
Wong E, Cuervo AM (2010) Autophagy gone awry in neurodegenerative diseases. Nat Neurosci 13(7):805–817
Mizushima N, Yoshimori T, Levine B (2010) Methods in mammalian autophagy research. Cell 140(3):313–326
Mizushima N, Yoshimori T (2007) How to interpret LC3 immunoblotting. Autophagy 3(6):542–545
Kaushik S, Massey A, Mizushima N, Cuervo AM (2008) Constitutive activation of chaperone-mediated autophagy in cells with impaired macroautophagy. Mol Biol Cell 19(5):2179–2192
Murphy DD, Rueter SM, Trojanowski JQ, Lee MY (2000) Synucleins are developmentally expressed, and α-synuclein regulates the size of the presynaptic vesicular pool in primary hippocampal neurons. J Neurosci 20(9):3214–3220
Yavich L, Tanila H, Vepsäläinen S, Jäkälä P (2004) Role of alpha-synuclein in presynaptic dopamine recruitment. J Neurosci 24(49):11165–11170
Abeliovich A, Schmitz Y, Farinas I, Choi-Lundberg D, Ho WH, Castillo PE, Shinsky N, Verdugo JM, Armanini M, Ryan A, Hynes M, Phillips H, Sulzer D, Rosenthal A (2000) Mice lacking alpha-synuclein display functional deficits in the nigrostriatal dopamine system. Neuron 29(1):235–252
Scott DA, Tabarean I, Tang Y, Cartier A, Masliah E, Roy S (2010) A pathologic cascade leading to synaptic dysfunction in alpha-synuclein-induced neurodegeneration. J Neurosci 30(24):8083–8095
Burré J, Sharma M, Tsetsenis T, Buchman V, Etherton MR, Südho TC (2010) α-Synuclein promotes SNARE-complex assembly in vivo and in vitro. Science 329(5999):1663–1667
Guerrero E, Vasudevaraju P, Hegde ML, Britton GB, Rao KS (2013) Recent advances in α-synuclein functions, advanced glycation, and toxicity: implications for Parkinson’s disease. Mol Neurobiol 47(2):525–536
Nemani VM, Lu W, Berge V, Nakamura K, Onoa B, Lee MK, Chaudhry FA, Nicoll RA, Edwards RH (2010) Increased expression of α-synuclein reduces neurotransmitter release by inhibiting synaptic vesicle reclustering after endocytosis. Neuron 65(1):66–79
Simón-Sánchez J, Schulte C, Bras JM et al (2009) Genome-wide association study reveals genetic risk underlying Parkinson’s disease. Nat Genet 41(12):1308–1312
Conway KA, Harper JD, Lansbury PT (1998) Accelerated in vitro fibril formation by a mutant a-synuclein linked to early-onset Parkinson disease. Nat Med 4(11):1318–1320
Narhi L, Wood SJ, Steavenson S, Jiang Y, Wu GM, Anafi D, Kaufman SA, Martin F, Sitney K, Denis P, Louis JC, Wypych J, Biere AL, Citron M (1999) Both familial Parkinson’s disease mutations accelerate a-synuclein aggregation. J Biol Chem 274(14):9843–9846
Lotharius J, Barg S, Wiekop P, Lundberg C, Raymon HK, Brundin P (2002) Effect of mutant α-synuclein on dopamine homeostasis in a new human mesencephalic cell line. J Biol Chem 277(41):38884–38894
Lee M, Hyun DH, Halliwell B, Jenner P (2001) Effect of overexpression of wild-type or mutant a-synuclein on cell susceptibility to insult. J Neurochem 76(4):998–1009
Bertoncini CW, Fernandez CO, Griesinger C, Jovin TM, Zweckstetter M (2005) Familial mutants of a-synuclein with increased neurotoxicity have a destabilized conformation. J Biol Chem 280(35):30649–30652
Giasson BI, Duda JE, Quinn SM, Zhang B, Trojanowski JQ, Lee VM (2002) Neuronal a-synucleinopathy with severe movement disorder in mice expressing A53T human a-synuclein. Neuron 34(4):521–533
Gispert S, Del Turco D, Garrett L, Chen A, Bernard DJ, Hamm-Clement J, Korf HW, Deller T, Braak H, Auburger G, Nussbaum RL (2003) Transgenic mice expressing mutant A53T human alpha-synuclein show neuronal dysfunction in the absence of aggregate formation. Mol Cell Neurosci 24(2):419–429
Gomez-Isla T, Irizarry MC, Mariash A, Cheung B, Soto O, Schrump S, Sondel J, Kotilinek L, Day J, Schwarzschild MA, Cha JH, Newell K, Miller DW, Uéda K, Young AB, Hyman BT, Ashe KH (2003) Motor dysfunction and gliosis with preserved dopaminergic markers in human a-synuclein A30P transgenic mice. Neurobiol Aging 24(2):245–258
Emmer KL, Waxman EA, Covy JP, Giasson BI (2011) E46K human a-synuclein transgenic mice develop Lewy-like and tau pathology associated with age-dependent, detrimental motor impairment. J Biol Chem 286(40):35104–35118
Hashimoto M, Hsu LJ, Rockenstein E, Takenouchi T, Mallory M, Masliah E (2002) α-Synuclein protects against oxidative stress via Inactivation of the C-Jun N-terminal Kinase stress-signaling pathway in neuronal cells. J. Biol Chem 277(13):11465–11472
Lashuel HA, Overk CR, Oueslati A, Masliah E (2013) The many faces of α-synuclein: from structure and toxicity to therapeutic target. Nat Rev Neurosci 14(1):38–48
Vekrellis K, Xilouri M, Emmanouilidou E, Rideout HJ, Stefanis L (2011) Pathological roles of α-synuclein in neurological disorders. Lancet Neurol 10(11):1015–1025
Vogiatzi T, Xilouri M, Vekrellis K, Stefanis L (2008) Wild type α-synuclein is degraded by chaperone-mediated autophagy and macroautophagy in neuronal cells. J Biol Chem 283(35):23542–23556
Ebrahimi-Fakhari D, Cantuti-Castelvetri I, Fan Z, Rockenstein E, Masliah E, Hyman BT, McLean PJ, Unni VK (2011) Distinct roles in vivo for the ubiquitin-proteasome system and theautophagy-lysosomal pathway in the degradation of α-synuclein. J Neurosci 31(41):14508–14520
Webb JL, Ravikular B, Atkins J, Skepper JN, Rubinsztein DC (2003) α-Synuclein is degraded by both autophagy and the proteasome. J Biol Chem 287(27):25009–25013
Ebrahimi-Fakhari D, Wahlster L, McLean PJ (2012) Protein degradation pathways in Parkinson’s disease: curse or blessing. Acta Neuropathol 124(2):153–172
Tai HC, Schuman EM (2008) Ubiquitin, the proteasome and protein degradation in neuronal function and dysfunction. Nat Rev Neurosci 9(11):826–838
Ciechanover A, Brundin P (2003) The ubiquitin proteasome system in neurodegenerative diseases: sometimes the chicken, sometimes the egg. Neuron 40(2):427–446
Cuervo AM, Dice JF (2000) Regulation of lamp2a levels in the lysosomal membrane. Traffic 1(7):570–583
Xilouri M, Brekk OR, Stefanis L (2013) Alpha-synuclein and protein degradation systems: a reciprocal relationship. Mol Neurobiol 47(2):537–551
Pandey N, Schmidt RE, Galvin JE (2006) The alpha-synuclein mutation E46K promotes aggregation in cultured cells. Exp Neurol 197(2):515–520
Fiske M, White M, Valtierra S, Herrera S, Solvang K, Konnikova A, DebBurman S (2011) Familial Parkinson’s disease E46K mutant α-synuclein localizes to membranous structures, forms aggregates, and induces toxicity in yeast models. ISRN Neurol 2011:521847
Rospigliosi CC, McClendon S, Schmid AW, Ramlall TF, Barré P, Lashuel HA, Eliezer D (2009) E46K Parkinson’s-linked mutation enhances C-terminal-to-N-terminal contacts in α-synuclein. J Mol Biol 388(5):1022–1032
Pan T, Rawal P, Wu Y, Xie W, Jankovic J, Le W (2009) Rapamycin protects against rotenone-induced apoptosis through autophagy induction. Neuroscience 164(2):541–551
Bove J, Perier C (2012) Neurotoxin-induced models of Parkinson’s disease. Neuroscience 211:51–76
Tanner CM, Kamel F, Ross GW, Hoppin JA, Goldman SM, Korell M, Marras C, Bhudhikanok GS, Kasten M, Chade AR, Comyns K, Richards MB, Meng C, Priestley B, Fernandez HH, Cambi F, Umbach DM, Blair A, Sandler DP, Langston JW (2011) Rotenone, paraquat, and Parkinson’s disease. Environ Health Perspect 119(6):866–872
Huang Y, Cheung L, Rowe D, Halliday G (2004) Genetic contributions to Parkinson’s disease. Brain Res Rev 46(1):44–70
Harris H, Rubinsztein DC (2011) Control of autophagy as a therapy for neurodegenerative disease. Nat Rev Neurol 8(2):108–117
Rubinsztein DC, Gestwicki JE, Murphy LO, Klionsky DJ (2007) Potential therapeutic applications of autophagy. Nat Rev Drug Discov 6(4):304–312
Berger Z, Ravikumar B, Menzies FM, Oroz LG, Underwood BR, Pangalos MN, Schmitt I, Wullner U, Evert BO, O'Kane CJ, Rubinsztein DC (2006) Rapamycin alleviates toxicity of different aggregate-prone proteins. Hum Mol Genet 15(3):433–442
Bové J, Martinez-Vicente M, Vila M (2011) Fighting neurodegeneration with rapamycin: mechanistic insights. Nat Rev Neurosci 12(8):437–452
Jiang TF, Zhang YJ, Zhou HY, Wang HM, Tian LP, Liu J, Ding JQ, Chen SD (2013) Curcumin ameliorates the neurodegenerative pathology in A53T α-synuclein cell model of Parkinson’s disease through the downregulation of mTOR/p70S6K signaling and the recovery of macroautophagy. J Neuroimmune Pharmacol 8(1):356–369
Sarkar S, Davies JE, Huang Z, Tunnacliffe A, Rubinsztein DC (2007) Trehalose, a novel mTOR-independent autophagy enhancer, accelerates the clearance of mutant huntingtin and α-synuclein. J Biol Chem 282(8):5641–5652
Pandey UB, Nie Z, Batlevi Y, McCray BA, Ritson GP, Nedelsky NB, Schwartz SL, DiProspero NA, Knight MA, Schuldiner O, Padmanabhan R, Hild M, Berry DL, Garza D, Hubbert CC, Yao TP, Baehrecke EH, Taylor JP (2007) HDAC6 rescues neurodegeneration and provides an essential link between autophagy and the UPS. Nature 447(7146):859–863
Tyedmers J, Mogk A, Bukau B (2010) Cellular strategies for controlling protein aggregation. Nat Rev Mol Cell Biol 11(11):777–788
Acknowledgments
This work was supported by the National Natural Science Foundation of China Grants (no. 81274122, 81102831, 81073078, 81373997, 81373510), the Special Purpose for New Drug Development (2012ZX09301002-004, 2012ZX09103101-006), the Studies on Structure and function of Bioactive Substances from Natural Medicines (IRT1007), the Beijing Natural Science Foundation (7131013), the Research Fund for the Doctoral Program of Higher Education of China (2012110613000), the Beijing Key Laboratory of New Drug Mechanisms and Pharmacological Evaluation Study (no. BZ0150), the PUMC Youth Fund, and the Fundamental Research Funds for the Central Universities. The authors thank Professor ZW Hu for presenting CHO cells.
Conflict of Interest
The authors report no biomedical financial interests or potential conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Fig. S1
Overexpression of WT α-synuclein has no effects on autophagy. (a) Stable PC12 cells overexpression of GFP, WT and E46K mutant α-synuclein were lysted. The detergent-soluble and detergent-insoluble fractions were subjected to SDS-PAGE. (b) HEK293 cells were transfected with WT, E46K mutant α-synuclein and an empty vector for 48 h. Cells were harvested and the detergent-soluble and detergent-insoluble fractions were subjected to SDS-PAGE. (c) Stable PC12 cells and HEK293 cells transfected with WT and E46K mutant α-synuclein were lysted and immunoblotted for LC3. (d) PC12 cells stably overexpressing WT α-synuclein-GFP were starved or not in EBSS for 4 h, then lysted and subjected to SDS-PAGE for analysis of the indicated proteins. (e) HEK293 cells were transfected with WT α-synuclein or an empty vector for 48 h followed by 4 h incubation with or not with EBSS. Indicated proteins were analyzed using western blotting. Data were shown as means ± SD for three independent experiments performed in triplicate. * P < 0.05, ** P < 0.01 compared with controls, # P < 0.05, ## P < 0.01 compared with WT α-synuclein by Two-tailed Student’s t- test. (GIF 103 kb)
Fig. S2
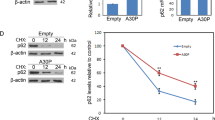
Overexpression of E46K mutant α-synuclein impairs ubiquitin-dependent degradation and enhances CMA activity. (a) PC12 cells stably overexpressing E46K mutant α-synuclein or GFP were lysted and immunoblotted for ubiquitin. (b) PC12 cells stably overexpressing E46K mutant α-synuclein or GFP were lysted. The detergent-insoluble fraction was solubilized and subjected to SDS-PAGE. (c) Immunostaining of ubiquitin in PC12 cells stably overexpressing E46K mutant α-synuclein and GFP. Images were acquired by a confocal microscopy. Scale bar: 10 μm. (d) PC12 cells stably overexpressing E46K mutant α-synuclein-GFP were treated with 10 μg/ml cycloheximide for the indicated periods, then lysted and subjected to immunoblotting for p53. (e) PC12 cells stably overexpressing E46K mutant α-synuclein-GFP were treated with 10 μg/ml cycloheximide for the indicated periods, then lysted and subjected to immunoblotting for GAPDH. Data were shown as means ± SD for three independent experiments performed in triplicate. * P < 0.05, ** P < 0.01 by Two-tailed Student’s t- test. (GIF 107 kb)
Rights and permissions
About this article
Cite this article
Yan, JQ., Yuan, YH., Gao, YN. et al. Overexpression of Human E46K Mutant α-Synuclein Impairs Macroautophagy via Inactivation of JNK1-Bcl-2 Pathway. Mol Neurobiol 50, 685–701 (2014). https://doi.org/10.1007/s12035-014-8738-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-014-8738-1