Abstract
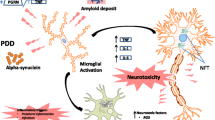
Although the central nervous system (CNS) has been defined as a privileged site in Alzheimer’s disease (AD), periphery can be more than simply witness of events leading to neurodegeneration. The CNS and peripheral blood can mutually communicate through cells and factors trafficking from the circulation into the brain and vice versa. A number of articles have reviewed inflammatory profiles and programmed cell death (PCD) in AD, separately in the CNS and at the peripheral level. This review does not provide an exhaustive account of what has been published on inflammation and PCD in AD. Rather, the aim of this review is to focus on possible linkages between the central and the peripheral compartments during AD progression, by critically analyzing, in a comparative manner, phenomena occurring in the CNS as well as the peripheral blood. In fact, growing evidence suggests that CNS and peripheral inflammation might present common features in the disease. Microarrays and metabolomics revealed that dysfunction of the glycolytic and oxidative pathways is similar in the brain and in the periphery. Moreover, dysregulated autophagosome/lysosomal molecular machinery, both at the CNS and the peripheral level, in AD-related cell damage, has been observed. Possible implications of these observations have been discussed.


Similar content being viewed by others
References
Glass CK, Saijo K, Winner B, Marchetto MC, Gage FH (2010) Mechanisms underlying inflammation in neurodegeneration. Cell 140:918–934. doi:10.1016/j.cell.2010.02.016
Gemechu JM, Bentivoglio M (2012) T cell recruitment in the brain during normal aging. Front Cell Neurosci 6:38. doi:10.3389/fncel.2012.00038
Mayeux R, Stern Y (2012) Epidemiology of Alzheimer disease. Cold Spring Harb Perspect Med 2. doi:10.1101/cshperspect.a006239
Haass C, Selkoe DJ (2007) Soluble protein oligomers in neurodegeneration: lessons from the Alzheimer’s amyloid beta-peptide. Nat Rev Mol Cell Biol 8:101–112. doi:10.1038/nrm2101
Selkoe DJ (2001) Alzheimer’s disease: genes, proteins, and therapy. Physiol Rev 81:741–766
Liu F, Iqbal K, Grundke-Iqbal I, Rossie S, Gong CX (2005) Dephosphorylation of tau by protein phosphatase 5: impairment in Alzheimer’s disease. J Biol Chem 280:1790–1796. doi:10.1074/jbc.M410775200
Woods NK, Padmanabhan J (2012) Neuronal calcium signaling and Alzheimer’s disease. Adv Exp Med Biol 740:1193–1217. doi:10.1007/978-94-007-2888-2_54
Leuner K, Muller WE, Reichert AS (2012) From mitochondrial dysfunction to amyloid beta formation: novel insights into the pathogenesis of Alzheimer’s disease. Mol Neurobiol 46:186–193. doi:10.1007/s12035-012-8307-4
Nixon RA (2013) The role of autophagy in neurodegenerative disease. Nat Med 19:983–997. doi:10.1038/nm.3232
Tan MS, Yu JT, Jiang T, Zhu XC, Tan L (2013) The NLRP3 inflammasome in Alzheimer’s Disease. Mol Neurobiol. doi:10.1007/s12035-013-8475-x
Niranjan R (2013) Molecular basis of etiological implications in Alzheimer’s disease: focus on neuroinflammation. Mol Neurobiol. doi:10.1007/s12035-013-8428-4
Tanzi RE (2012) The genetics of Alzheimer disease. Cold Spring Harb Perspect Med. doi:10.1101/cshperspect.a006296
Akiyama H, Barger S, Barnum S, Bradt B, Bauer J, Cole GM, Cooper NR, Eikelenboom P, Emmerling M, Fiebich BL, Finch CE, Frautschy S, Griffin WS, Hampel H, Hull M, Landreth G, Lue L, Mrak R, Mackenzie IR, McGeer PL, O’Banion MK, Pachter J, Pasinetti G, Plata-Salaman C, Rogers J, Rydel R, Shen Y, Streit W, Strohmeyer R, Tooyoma I, Van Muiswinkel FL, Veerhuis R, Walker D, Webster S, Wegrzyniak B, Wenk G, Wyss-Coray T (2000) Inflammation and Alzheimer’s disease. Neurobiol Aging 21:383–421. doi:10.1016/s0197-4580(00)00124-x
Liu S, Liu Y, Hao W, Wolf L, Kiliaan AJ, Penke B, Rube CE, Walter J, Heneka MT, Hartmann T, Menger MD, Fassbender K (2012) TLR2 is a primary receptor for Alzheimer’s amyloid beta peptide to trigger neuroinflammatory activation. J Immunol 188:1098–1107. doi:10.4049/jimmunol.1101121
Reddy PH, McWeeney S, Park BS, Manczak M, Gutala RV, Partovi D, Jung Y, Yau V, Searles R, Mori M, Quinn J (2004) Gene expression profiles of transcripts in amyloid precursor protein transgenic mice: up-regulation of mitochondrial metabolism and apoptotic genes is an early cellular change in Alzheimer’s disease. Hum Mol Genet 13:1225–1240. doi:10.1093/hmg/ddh140
Brooks WM, Lynch PJ, Ingle CC, Hatton A, Emson PC, Faull RL, Starkey MP (2007) Gene expression profiles of metabolic enzyme transcripts in Alzheimer’s disease. Brain Res 1127:127–135. doi:10.1016/j.brainres.2006.09.106
Leuner K, Schutt T, Kurz C, Eckert SH, Schiller C, Occhipinti A, Mai S, Jendrach M, Eckert GP, Kruse SE, Palmiter RD, Brandt U, Drose S, Wittig I, Willem M, Haass C, Reichert AS, Muller WE (2012) Mitochondrion-derived reactive oxygen species lead to enhanced amyloid beta formation. Antioxid Redox Signal 16:1421–1433. doi:10.1089/ars.2011.4173
Howells C, Saar K, Eaton E, Ray S, Palumaa P, Shabala L, Adlard PA, Bennett W, West AK, Guillemin GJ, Chung RS (2012) Redox-active Cu(II)-A beta causes substantial changes in axonal integrity in cultured cortical neurons in an oxidative-stress dependent manner. Exp Neurol 237:499–506. doi:10.1016/j.expneuro1.2012.06.002
Terai K, Matsuo A, McGeer PL (1996) Enhancement of immunoreactivity for NF-kappa B in the hippocampal formation and cerebral cortex of Alzheimer’s disease. Brain Res 735:159–168
Chami L, Buggia-Prevot V, Duplan E, Delprete D, Chami M, Peyron JF, Checler F (2012) Nuclear factor-kappaB regulates betaAPP and beta- and gamma-secretases differently at physiological and supraphysiological Abeta concentrations. J Biol Chem 287:24573–24584. doi:10.1074/jbc.M111.333054
Nam JH, Park KW, Park ES, Lee YB, Lee HG, Baik HH, Kim YS, Maeng S, Park J, Jin BK (2012) Interleukin-13/-4-induced oxidative stress contributes to death of hippocampal neurons in abeta1-42-treated hippocampus in vivo. Antioxid Redox Signal 16:1369–1383. doi:10.1089/ars.2011.4175
Maes OC, Xu S, Yu B, Chertkow HM, Wang E, Schipper HM (2007) Transcriptional profiling of Alzheimer blood mononuclear cells by microarray. Neurobiol Aging 28:1795–1809. doi:10.1016/j.neurobiolaging.2006.08.004
Booij BB, Lindahl T, Wetterberg P, Skaane NV, Saebo S, Feten G, Rye PD, Kristiansen LI, Hagen N, Jensen M, Bardsen K, Winblad B, Sharma P, Lonneborg A (2011) A gene expression pattern in blood for the early detection of Alzheimer’s disease. J Alzheimers Dis 23:109–119. doi:10.3233/JAD-2010-101518
Lunnon K, Ibrahim Z, Proitsi P, Lourdusamy A, Newhouse S, Sattlecker M, Furney S, Saleem M, Soininen H, Kloszewska I, Mecocci P, Tsolaki M, Vellas B, Coppola G, Geschwind D, Simmons A, Lovestone S, Dobson R, Hodges A, AddNeuroMed C (2012) Mitochondrial dysfunction and immune activation are detectable in early Alzheimer’s disease blood. J Alzheimers Dis 30:685–710. doi:10.3233/jad-2012-111592
Sultana R, Mecocci P, Mangialasche F, Cecchetti R, Baglioni M, Butterfield DA (2011) Increased protein and lipid oxidative damage in mitochondria isolated from lymphocytes from patients with Alzheimer’s disease: insights into the role of oxidative stress in Alzheimer’s disease and initial investigations into a potential biomarker for this dementing disorder. J Alzheimers Dis 24:77–84. doi:10.3233/jad-2011-101425
Torres LL, Quaglio NB, de Souza GT, Garcia RT, Dati LM, Moreira WL, Loureiro AP, de Souza-Talarico JN, Smid J, Porto CS, Bottino CM, Nitrini R, Barros SB, Camarini R, Marcourakis T (2011) Peripheral oxidative stress biomarkers in mild cognitive impairment and Alzheimer’s disease. J Alzheimers Dis 26:59–68. doi:10.3233/jad-2011-110284
Trushina E, Dutta T, Persson XM, Mielke MM, Petersen RC (2013) Identification of altered metabolic pathways in plasma and CSF in mild cognitive impairment and Alzheimer’s disease using metabolomics. PLoS One 8:e63644. doi:10.1371/journal.pone.0063644
Lombardi VR, Garcia M, Rey L, Cacabelos R (1999) Characterization of cytokine production, screening of lymphocyte subset patterns and in vitro apoptosis in healthy and Alzheimer’s disease (AD) individuals. J Neuroimmunol 97:163–171. doi:10.1016/s0165-5728(99)00046-6
Sala G, Galimberti G, Canevari C, Raggi ME, Isella V, Facheris M, Appollonio I, Ferrarese C (2003) Peripheral cytokine release in Alzheimer patients: correlation with disease severity. Neurobiol Aging 24:909–914. doi:10.1016/s0197-4580(03)00010-1
Licastro F, Pedrini S, Caputo L, Annoni G, Davis LJ, Ferri C, Casadei V, Grimaldi LM (2000) Increased plasma levels of interleukin-1, interleukin-6 and alpha-1-antichymotrypsin in patients with Alzheimer’s disease: peripheral inflammation or signals from the brain? J Neuroimmunol 103:97–102. doi:10.1016/s0165-5728(99)00226-x
Swardfager W, Lanctot K, Rothenburg L, Wong A, Cappell J, Herrmann N (2010) A meta-analysis of cytokines in Alzheimer’s disease. Biol Psychiatry 68:930–941. doi:10.1016/j.biopsych.2010.06.012
Bossu P, Ciaramella A, Salani F, Bizzoni F, Varsi E, Di Iulio F, Giubilei F, Gianni W, Trequattrini A, Moro ML, Bernardini S, Caltagirone C, Spalletta G (2008) Interleukin-18 produced by peripheral blood cells is increased in Alzheimer’s disease and correlates with cognitive impairment. Brain Behav Immun 22:487–492. doi:10.1016/j.bbi.2007.10.001
Ascolani A, Balestrieri E, Minutolo A, Mosti S, Spalletta G, Bramanti P, Mastino A, Caltagirone C, Macchi B (2012) Dysregulated NF-kappa B pathway in peripheral mononuclear cells of Alzheimer’s disease patients. Curr Alzheimer Res 9:128–137. doi:10.2174/156720512799015091
Mecocci P, Cherubini A, Senin U (1997) Increased oxidative damage in lymphocytes of Alzheimer’s disease patients. J Am Geriatr Soc 45:1536–1537
Mecocci P, Polidori MC, Cherubini A, Ingegni T, Mattioli P, Catani M, Rinaldi P, Cecchetti R, Stahl W, Senin U, Beal MF (2002) Lymphocyte oxidative DNA damage and plasma antioxidants in Alzheimer disease. Arch Neurol 59:794–798. doi:10.1001/archneur.59.5.794
Morocz M, Kalman J, Juhasz A, Sinko I, McGlynn AP, Downes CS, Janka Z, Rasko I (2002) Elevated levels of oxidative DNA damage in lymphocytes from patients with Alzheimer’s disease. Neurobiol Aging 23:47–53. doi:10.1016/s0197-4580(01)00257-3
Leutner S, Schindowski K, Frolich L, Maurer K, Kratzsch T, Eckert A, Muller WE (2005) Enhanced ROS-generation in lymphocytes from Alzheimer’s patients. Pharmacopsychiatry 38:312–315. doi:10.1055/s-2005-916186
Kadioglu E, Sardas S, Aslan S, Isik E, Esat Karakaya A (2004) Detection of oxidative DNA damage in lymphocytes of patients with Alzheimer’s disease. Biomarkers 9:203–209. doi:10.1080/13547500410001728390
Baldeiras I, Santana I, Proenca MT, Garrucho MH, Pascoal R, Rodrigues A, Duro D, Oliveira CR (2008) Peripheral oxidative damage in mild cognitive impairment and mild Alzheimer’s disease. J Alzheimers Dis 15:117–128
Straface E, Matarrese P, Gambardella L, Vona R, Sgadari A, Silveri MC, Malorni W (2005) Oxidative imbalance and cathepsin D changes as peripheral blood biomarkers of Alzheimer disease: a pilot study. FEBS Lett 579:2759–2766. doi:10.1016/j.febslet.2005.03.094
Leuner K, Schulz K, Schutt T, Pantel J, Prvulovic D, Rhein V, Savaskan E, Czech C, Eckert A, Muller WE (2012) Peripheral mitochondrial dysfunction in Alzheimer’s disease: focus on lymphocytes. Mol Neurobiol 46:194–204. doi:10.1007/s12035-012-8300-y
Sultana R, Baglioni M, Cecchetti R, Cai J, Klein JB, Bastiani P, Ruggiero C, Mecocci P, Butterfield DA (2013) Lymphocyte mitochondria: toward identification of peripheral biomarkers in the progression of Alzheimer disease. Free Radic Biol Med 65C:595–606. doi:10.1016/j.freeradbiomed.2013.08.001
Martin SJ, Henry CM, Cullen SP (2012) A perspective on mammalian caspases as positive and negative regulators of inflammation. Mol Cell 46:387–397. doi:10.1016/j.molcel.2012.04.026
Raina AK, Hochman A, Zhu X, Rottkamp CA, Nunomura A, Siedlak SL, Boux H, Castellani RJ, Perry G, Smith MA (2001) Abortive apoptosis in Alzheimer’s disease. Acta Neuropathol 101:305–310. doi:10.1007/s004010100378
Rohn TT, Head E, Nesse WH, Cotman CW, Cribbs DH (2001) Activation of caspase-8 in the Alzheimer’s disease brain. Neurobiol Dis 8:1006–1016. doi:10.1006/nbdi.2001.0449
Pompl PN, Yemul S, Xiang ZM, Ho L, Haroutunian V, Purohit D, Mohs R, Pasinetti GM (2003) Caspase gene expression in the brain as a function of the clinical progression of Alzheimer disease. Arch Neurol 60:369–376. doi:10.1001/archneur.60.3.369
Gamblin TC, Chen F, Zambrano A, Abraha A, Lagalwar S, Guillozet AL, Lu M, Fu Y, Garcia-Sierra F, LaPointe N, Miller R, Berry RW, Binder LI, Cryns VL (2003) Caspase cleavage of tau: linking amyloid and neurofibrillary tangles in Alzheimer’s disease. Proc Natl Acad Sci U S A 100:10032–10037. doi:10.1073/pnas.1630428100
Gervais FG, Xu D, Robertson GS, Vaillancourt JP, Zhu Y, Huang J, LeBlanc A, Smith D, Rigby M, Shearman MS, Clarke EE, Zheng H, Van Der Ploeg LH, Ruffolo SC, Thornberry NA, Xanthoudakis S, Zamboni RJ, Roy S, Nicholson DW (1999) Involvement of caspases in proteolytic cleavage of Alzheimer’s amyloid-beta precursor protein and amyloidogenic A beta peptide formation. Cell 97:395–406. doi:10.1016/s0092-8674(00)80748-5
Rissman RA, Poon WW, Blurton-Jones M, Oddo S, Torp R, Vitek MP, LaFerla FM, Rohn TT, Cotman CW (2004) Caspase-cleavage of tau is an early event in Alzheimer disease tangle pathology. J Clin Invest 114:121–130. doi:10.1172/jc1200420640
Oddo S, Caccamo A, Shepherd JD, Murphy MP, Golde TE, Kayed R, Metherate R, Mattson MP, Akbari Y, LaFerla FM (2003) Triple-transgenic model of Alzheimer’s disease with plaques and tangles: intracellular A beta and synaptic dysfunction. Neuron 39:409–421. doi:10.1016/S0896-6273(03)00434-3
Oddo S, Caccamo A, Kitazawa M, Tseng BP, LaFerla FM (2003) Amyloid deposition precedes tangle formation in a triple transgenic model of Alzheimer’s disease. Neurobiol Aging 24:1063–1070. doi:10.1016/j.neurobiolaging.2003.08.012
Guo H, Albrecht S, Bourdeau M, Petzke T, Bergeron C, LeBlanc AC (2004) Active caspase-6 and caspase-6-cleaved tau in neuropil threads, neuritic plaques, and neurofibrillary tangles of Alzheimer’s disease. Am J Pathol 165:523–531. doi:10.1016/S0002-9440(10)63317-2
Albrecht S, Bourdeau M, Bennett D, Mufson EJ, Bhattacharjee M, LeBlanc AC (2007) Activation of caspase-6 in aging and mild cognitive impairment. Am J Pathol 170:1200–1209. doi:10.2353/ajpath.2007.060974
Spires TL, Orne JD, SantaCruz K, Pitstick R, Carlson GA, Ashe KH, Hyman BT (2006) Region-specific dissociation of neuronal loss and neurofibrillary pathology in a mouse model of tauopathy. Am J Pathol 168:1598–1607. doi:10.2353/ajpath.2006.050840
Morsch R, Simon W, Coleman PD (1999) Neurons may live for decades with neurofibrillary tangles. J Neuropath Exp Neur 58:188–197. doi:10.1097/00005072-199902000-00008
Spires-Jones TL, de Calignon A, Matsui T, Zehr C, Pitstick R, Wu HY, Osetek JD, Jones PB, Bacskai BJ, Feany MB, Carlson GA, Ashe KH, Lewis J, Hyman BT (2008) In vivo imaging reveals dissociation between caspase activation and acute neuronal death in tangle-bearing neurons. J Neurosci 28:862–867. doi:10.1523/jneurosci.3072-08.2008
de Calignon A, Fox LM, Pitstick R, Carlson GA, Bacskai BJ, Spires-Jones TL, Hyman BT (2010) Caspase activation precedes and leads to tangles. Nature 464:1201–1204. doi:10.1038/nature08890
Burguillos MA, Deierborg T, Kavanagh E, Persson A, Hajji N, Garcia-Quintanilla A, Cano J, Brundin P, Englund E, Venero JL, Joseph B (2011) Caspase signalling controls microglia activation and neurotoxicity. Nature 472:319–324. doi:10.1038/nature09788
D’Amelio M, Cavallucci V, Middei S, Marchetti C, Pacioni S, Ferri A, Diamantini A, De Zio D, Carrara P, Battistini L, Moreno S, Bacci A, Ammassari-Teule M, Marie H, Cecconi F (2011) Caspase-3 triggers early synaptic dysfunction in a mouse model of Alzheimer’s disease. Nat Neurosci 14:69–76. doi:10.1038/nn.2709
Rohn TT, Wirawan E, Brown RJ, Harris JR, Masliah E, Vandenabeele P (2011) Depletion of Beclin-1 due to proteolytic cleavage by caspases in the Alzheimer’s disease brain. Neurobiol Dis 43:68–78. doi:10.1016/j.nbd.2010.11.003
Jaeger PA, Pickford F, Sun CH, Lucin KM, Masliah E, Wyss-Coray T (2010) Regulation of amyloid precursor protein processing by the Beclin 1 complex. PLoS One 5:e11102. doi:10.1371/journal.pone.0011102
Hamano T, Gendron TF, Causevic E, Yen SH, Lin WL, Isidoro C, Deture M, Ko LW (2008) Autophagic-lysosomal perturbation enhances tau aggregation in transfectants with induced wild-type tau expression. Eur J Neurosci 27:1119–1130. doi:10.1111/j.1460-9568.2008.06084.x
Wang C, Yu JT, Miao D, Wu ZC, Tan MS, Tan L (2013) Targeting the mTOR signaling network for Alzheimer’s disease therapy. Mol Neurobiol. doi:10.1007/s12035-013-8505-8
Zhu XC, Yu JT, Jiang T, Tan L (2013) Autophagy modulation for Alzheimer’s disease therapy. Mol Neurobiol. doi:10.1007/s12035-013-8457-z
Cai Z, Yan LJ (2013) Rapamycin, autophagy, and Alzheimer’s disease. J Biochem Pharmacol Res 1:84–90
Zheng L, Terman A, Hallbeck M, Dehvari N, Cowburn RF, Benedikz E, Kagedal K, Cedazo-Minguez A, Marcusson J (2011) Macroautophagy-generated increase of lysosomal amyloid beta-protein mediates oxidant-induced apoptosis of cultured neuroblastoma cells. Autophagy 7:1528–1545
Wang H, Ma J, Tan Y, Wang Z, Sheng C, Chen S, Ding J (2010) Amyloid-beta1-42 induces reactive oxygen species-mediated autophagic cell death in U87 and SH-SY5Y cells. J Alzheimers Dis 21:597–610. doi:10.3233/jad-2010-091207
Sanchez-Varo R, Trujillo-Estrada L, Sanchez-Mejias E, Torres M, Baglietto-Vargas D, Moreno-Gonzalez I, De Castro V, Jimenez S, Ruano D, Vizuete M, Davila JC, Garcia-Verdugo JM, Jimenez AJ, Vitorica J, Gutierrez A (2012) Abnormal accumulation of autophagic vesicles correlates with axonal and synaptic pathology in young Alzheimer’s mice hippocampus. Acta Neuropathol 123:53–70. doi:10.1007/s00401-011-0896-x
Tung YT, Wang BJ, Hu MK, Hsu WM, Lee H, Huang WP, Liao YF (2012) Autophagy: a double-edged sword in Alzheimer’s disease. J Biosci 37:157–165
Tacconi S, Perri R, Balestrieri E, Grelli S, Bernardini S, Annichiarico R, Mastino A, Caltagirone C, Macchi B (2004) Increased caspase activation in peripheral blood mononuclear cells of patients with Alzheimer’s disease. Exp Neurol 190:254–262. doi:10.1016/j.expneurol.2004.07.009
Blandini F, Sinforiani E, Pacchetti C, Samuele A, Bazzini E, Zangaglia R, Nappi G, Martignoni E (2006) Peripheral proteasome and caspase activity in Parkinson disease and Alzheimer disease. Neurology 66:529–534. doi:10.1212/01.wnl.0000198511.09968.b3
Cosentino M, Colombo C, Mauri M, Ferrari M, Corbetta S, Marino F, Bono G, Lecchini S (2009) Expression of apoptosis-related proteins and of mRNA for dopaminergic receptors in peripheral blood mononuclear cells from patients with Alzheimer disease. Alzheimer Dis Assoc Disord 23:88–90
Pellicano M, Larbi A, Goldeck D, Colonna-Romano G, Buffa S, Bulati M, Rubino G, Iemolo F, Candore G, Caruso C, Derhovanessian E, Pawelec G (2012) Immune profiling of Alzheimer patients. J Neuroimmunol 242:52–59. doi:10.1016/j.jneuroim.2011.11.005
Zlokovic BV, Ghiso J, Mackic JB, McComb JG, Weiss MH, Frangione B (1993) Blood-brain barrier transport of circulating Alzheimer’s amyloid beta. Biochem Biophys Res Commun 197:1034–1040. doi:10.1006/bbrc.1993.2582
Schindowski K, Peters J, Gorriz C, Schramm U, Weinandi T, Leutner S, Maurer K, Frolich L, Muller WE, Eckert A (2006) Apoptosis of CD4+ T and natural killer cells in Alzheimer’s disease. Pharmacopsychiatry 39:220–228. doi:10.1055/s-2006-954591
Eckert A, Oster M, Zerfass R, Hennerici M, Muller WE (2001) Elevated levels of fragmented DNA nucleosomes in native and activated lymphocytes indicate an enhanced sensitivity to apoptosis in sporadic Alzheimer’s disease. Specific differences to vascular dementia. Dement Geriatr Cogn Disord 12:98–105. doi:10.1159/000051242
Gatta L, Cardinale A, Wannenes F, Consoli C, Armani A, Molinari F, Mammi C, Stocchi F, Torti M, Rosano GM, Fini M (2009) Peripheral blood mononuclear cells from mild cognitive impairment patients show deregulation of Bax and Sod1 mRNAs. Neurosci Lett 453:36–40. doi:10.1016/j.neulet.2009.02.003
Zana M, Juhasz A, Rimanoczy A, Bjelik A, Baltas E, Ocsovszki I, Boda K, Penke B, Dobozy A, Kemeny L, Janka Z, Kalman J (2006) Alzheimer’s lymphocytes are resistant to ultraviolet B-induced apoptosis. Neurobiol Aging 27:831–834. doi:10.1016/j.neurobiolaging.2005.04.007
Yates SC, Zafar A, Paul H, Nagy S, Durant S, Bicknell R, Wilcock G, Christie S, Esiri MM, Smith AD, Nagy Z (2013) Dysfunction of the mTOR pathway is a risk factor for Alzheimer disease. Acta Neuropathol Commun 1. doi:10.1186/2051-5960-1-3
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Macchi, B., Marino-Merlo, F., Frezza, C. et al. Inflammation and Programmed Cell Death in Alzheimer’s Disease: Comparison of the Central Nervous System and Peripheral Blood. Mol Neurobiol 50, 463–472 (2014). https://doi.org/10.1007/s12035-014-8641-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-014-8641-9