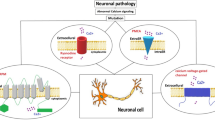
Abstract
Autism spectrum disorder (ASD) is a severe, complex neurodevelopmental disorder characterized by impairments in reciprocal social interaction and communication, and restricted and stereotyped patterns of interests and behaviors. Recent evidence has unveiled an important role for calcium (Ca2+) signaling in the pathogenesis of ASD. Post-mortem studies of autistic brains have pointed toward abnormalities in mitochondrial function as possible downstream consequences of altered Ca2+ signaling, abnormal synapse formation, and dysreactive immunity. SLC25A12, an ASD susceptibility gene, encodes the Ca2+-regulated mitochondrial aspartate–glutamate carrier, isoform 1 (AGC1). AGC1 is an important component of the malate/aspartate shuttle, a crucial system supporting oxidative phosphorylation and adenosine triphosphate (ATP) production. Here, we review the physiological roles of AGC1, its links to calcium homeostasis, and its involvement in autism pathogenesis.



Similar content being viewed by others
References
Rimessi A, Giorgi C, Pinton P, Rizzuto R (2008) The versatility of mitochondrial calcium signals: from stimulation of cell metabolism to induction of cell death. Biochim Biophys Acta 1777:808–816
Gunter KK, Gunter TE (1994) Transport of calcium by mitochondria. J Bioenerg Biomembr 26:471–485
Gunter TE, Yule DI, Gunter KK, Eliseev RA, Salter JD (2004) Calcium and mitochondria. FEBS Lett 567:96–102
Kirichok Y, Krapivinsky G, Clapham DE (2004) The mitochondrial calcium uniporter is a highly selective ion channel. Nature 427:360–364
McCormack JG, Halestrap AP, Denton RM (1990) Role of calcium ions in regulation of mammalian intramitochondrial metabolism. Physiol Rev 70:391–425
Balaban RS (2002) Cardiac energy metabolism homeostasis: role of cytosolic calcium. J Mol Cell Cardiol 34:1259–1271
Wernette ME, Ochs RS, Lardy HA (1981) Ca2+ stimulation of rat liver mitochondrial glycerophosphate dehydrogenase. J Biol Chem 256:12767–12771
Rutter GA, Pralong WF, Wollheim CB (1992) Regulation of mitochondrial glycerol-phosphate dehydrogenase by Ca2+ within electropermeabilized insulin-secreting cells (INS-1). Biochim Biophys Acta 1175:107–113
MacDonald MJ, Brown LJ (1996) Calcium activation of mitochondrial glycerol phosphate dehydrogenase restudied. Arch Biochem Biophys 326:79–84
Celsi F, Pizzo P, Brini M, Leo S, Fotino C, Pinton P, Rizzuto R (2009) Mitochondria, calcium and cell death: a deadly triad in neurodegeneration. Biochim Biophys Acta 1787:335–344
Krey JF, Dolmetsch RE (2007) Molecular mechanisms of autism: a possible role for Ca2+ signaling. Curr Opin Neurobiol 17:112–119
Palmieri L, Persico AM (2010) Mitochondrial dysfunction in autism spectrum disorders: cause or effect? Biochim Biophys Acta 1797:1130–1137
Palmieri L, Pardo B, Lasorsa FM, del Arco A, Kobayashi K, Iijima M, Runswick MJ, Walker JE, Saheki T, Satrústegui J, Palmieri F (2001) Citrin and aralar1 are Ca(2+)-stimulated aspartate/glutamate transporters in mitochondria. EMBO J 20:5060–5069
Del Arco A, Morcillo J, Martínez-Morales JR, Galián C, Martos V, Bovolenta P, Satrústegui J (2002) Expression of the aspartate/glutamate mitochondrial carriers aralar1 and citrin during development and in adult rat tissues. Eur J Biochem 269:3313–3320
Contreras L, Urbieta A, Kobayashi K, Saheki T, Satrústegui J (2010) Low levels of citrin (SLC25A13) expression in adult mouse brain restricted to neuronal clusters. J Neurosci Res 88:1009–1016
Del Arco A, Satrústegui J (1998) Molecular cloning of Aralar, a new member of the mitochondrial carrier superfamily that binds calcium and is present in human muscle and brain. J Biol Chem 273:23327–23334
Lasorsa FM, Pinton P, Palmieri L, Fiermonte G, Rizzuto R, Palmieri F (2003) Recombinant expression of the Ca(2+)-sensitive aspartate/glutamate carrier increases mitochondrial ATP production in agonist-stimulated Chinese hamster ovary cells. J Biol Chem 278:38686–38692
Palmieri F (2004) The mitochondrial transporter family (SLC25): physiological and pathological implications. Pflugers Arch 447:689–709
Borst P (1963) Functionelle und Morphologische Organisation der Zelle. In: Karlson P (ed). Springer, Berlin
Sadava D, Depper M, Gilbert M, Bernard B, McCabe ER (1987) Development of enzymes of glycerol metabolism in human fetal liver. Biol Neonate 52:26–32
Taylor WT, Fahy E, Zhang B, Glenn GM, Warnock DE, Wiley S, Murphy AN, Gaucher SP, Capaldi RA, Gibson BW, Ghosh SS (2003) Characterization of the human heart mitochondrial proteome. Nat Biotechnol 21:281–286
Scholz TD, Koppenhafer SL (1995) Reducing equivalent shuttles in developing porcine myocardium: enhanced capacity in the newborn heart. Pediatr Res 38:221–227
Scholz TD, Koppenhafer SL, tenEyck CJ, Schutte BC (1998) Ontogeny of malate–aspartate shuttle capacity and gene expression in cardiac mitochondria. Am J Physiol Cell Physiol 274:780–788
Bassani RA, Fagian MM, Bassani JW, Vercesi AE (1998) Changes in calcium uptake rate by rat cardiac mitochondria during postnatal development. J Mol Cell Cardiol 30:2013–2023
Begum L, Jalil MA, Kobayashi K, Iijima M, Li MX, Yasuda T, Horiuchi M, del Arco A, Satrustegui J, Saheki T (2002) Expression of three mitochondrial solute carriers, citrin, aralar1 and ornithine transporter, in relation to urea cycle in mice. Biochim Biophys Acta 1574:283–292
Dukes ID, McIntyre MS, Mertz RJ, Philipson LH, Roe MW, Spencer B, Worley JF 3rd (1994) Dependence on NADH produced during glycolysis for beta-cell glucose signaling. J Biol Chem 269:10979–10982
Giroix MH, Rasschaert J, Bailbe D, Leclercq-Meyer V, Sener A, Portha B, Malaisse WJ (1991) Impairment of glycerol phosphate shuttle in islets from rats with diabetes induced by neonatal streptozocin. Diabetes 40:227–232
Tan C, Tuch BE, Tu J, Brown SA (2002) Role of NADH shuttles in glucose-induced insulin secretion from fetal beta-cells. Diabetes 51:2989–2996
Eto K, Tsubamoto Y, Terauchi Y, Sugiyama T, Kishimoto T, Takahashi N, Yamauchi N, Kubota N, Murayama S, Aizawa T, Akanuma Y, Aizawa S, Kasai H, Yazaki Y, Kadowaki T (1999) Role of NADH shuttle system in glucose-induced activation of mitochondrial metabolism and insulin secretion. Science 283:981–985
Rubi B, del Arco A, Bartley C, Satrustegui J, Maechler P (2004) The malate–aspartate NADH shuttle member Aralar1 determines glucose metabolic fate, mitochondrial activity, and insulin secretion in beta cells. J Biol Chem 279:55659–55666
Mármol P, Pardo B, Wiederkehr A, del Arco A, Wollheim CB, Satrústegui J (2009) Requirement for aralar and its Ca2+-binding sites in Ca2+ signal transduction in mitochondria from INS-1 clonal beta-cells. J Biol Chem 284:515–524
Lane M, Gardner DK (2000) Lactate regulates pyruvate uptake and metabolism in the preimplantation mouse embryo. Biol Reprod 62:16–22
Lane M, Gardner DK (2005) Mitochondrial malate–aspartate shuttle regulates mouse embryo nutrient consumption. J Biol Chem 280:18361–18367
Ramos M, del Arco A, Pardo B, Martinez-Serrano A, Martinez-Morales JR, Kobayashi K, Yasuda T, Bogonez E, Bovolenta P, Saheki T, Satrùstegui J (2003) Developmental changes in the Ca2+-regulated mitochondrial aspartate–glutamate carrier aralar1 in brain and prominent expression in the spinal cord. Dev Brain Res 143:33–46
Cheeseman AJ, Clark JB (1988) Influence of the malate–aspartate shuttle on oxidative metabolism in synaptosomes. J Neurochem 50:1559–1565
McKenna MC, Tildon JT, Stevenson JH, Boatright R, Huang S (1993) Regulation of energy metabolism in synaptic terminals and cultured rat brain astrocytes: differences revealed using aminooxyacetate. Dev Neurosci 15:320–329
Jalil MA, Begum L, Contreras L, Pardo B, Iijima M, Li MX, Ramos M, Marmol P, Horiuchi M, Shimotsu K, Nakagawa S, Okubo A, Sameshima M, Isashiki Y, del Arco A, Kobayashi K, Satrùstegui J, Saheki T (2005) Reduced N-acetylaspartate levels in mice lacking aralar, a brain and muscle-type mitochondrial aspartate–glutamate carrier. J Biol Chem 280:31333–31339
D’Adamo AF Jr, Yatsu FM (1966) Acetate metabolism in the nervous system. N-acetyl-L-aspartic acid and the biosynthesis of brain lipids. J Neurochem 13:961–965
Burri R, Steffen C, Herschkowitz N (1991) N-acetyl-aspartate is a major source of acetyl groups for lipid synthesis during rat brain development. Dev Neurosci 13:403–411
Mehta V, Namboodiri MA (1995) N-acetylaspartate as an acetyl source in the nervous system. Brain Res Mol Brain Res 31:151–157
Chakraborty G, Mekala P, Yahya D, Wu G, Ledeen RW (2001) Intraneuronal N-acetylaspartate supplies acetyl groups for myelin lipid synthesis: evidence for myelin-associated aspartoacylase. J Neurochem 78:736–745
Wibom R, Lasorsa F, Töhönen V, Barbaro M, Sterky F, Kucinski T, Naess K, Jonsson M, Pierri C, Palmieri F, Wedell A (2009) AGC1 deficiency associated with global cerebral hypomyelination. N Engl J Med 361:489–495
Heineman FW, Balaban RS (1990) Phosphorus-31 nuclear magnetic resonance analysis of transient changes of canine myocardial metabolism in vivo. J Clin Invest 85:843–852
Sharma N, Okere IC, Brunengraber DZ, McElfresh TA, King KL, Sterk JP, Huang H, Chandler MP, Stanley WC (2005) Regulation of pyruvate dehydrogenase activity and citric acid cycle intermediates during high cardiac power generation. J Physiol 562:593–603
Hansford RG, Zorov D (1998) Role of mitochondrial calcium transport in the control of substrate oxidation. Mol Cell Biochem 184:359–369
Territo PR, Mootha VK, French SA, Balaban RS (2000) Ca(2+) activation of heart mitochondrial oxidative phosphorylation: role of the F(0)/F(1)-ATPase. Am J Physiol Cell Physiol 278:423–435
Korzeniewski B (2007) Regulation of oxidative phosphorylation through parallel activation. Biophys Chem 129:93–110
Rutter GA, Denton RM (1988) Regulation of NAD+-linked isocitrate dehydrogenase and 2-oxoglutarate dehydrogenase by Ca2+ ions within toluene-permeabilized rat heart mitochondria. Interactions with regulation by adenine nucleotides and NADH/NAD+ ratios. Biochem J 252:181–189
Rutter GA, Midgley PJ, Denton RM (1989) Regulation of the pyruvate dehydrogenase complex by Ca2+ within toluene-permeabilized heart mitochondria. Biochim Biophys Acta 1014:263–270
Das AM (2003) Regulation of the mitochondrial ATP-synthase in health and disease. Mol Genet Metab 79:71–82
Gellerich FN, Gizatullina Z, Nguyen HP, Trumbeckaite S, Vielhaber S, Seppet E, Zierz S, Landwehrmeyer B, Ries O, von Hoersten S, Striggow F (2008) Impaired regulation of brain mitochondria by extramitochondrial Ca2+ in transgenic Huntington disease rats. J Biol Chem 283:30715–30724
Gellerich FN, Gizatullina Z, Arandacikaite O, Jerzembeck D, Vielhaber S, Seppet E, Striggow F (2009) Extramitochondrial Ca2+ in the nanomolar range regulates glutamate-dependent oxidative phosphorylation on demand. PloS One 4:e8181
Contreras L, Gomez-Puertas P, Iijima M, Kobayashi K, Saheki T, Satrústegui J (2007) Ca2+ activation kinetics of the two aspartate–glutamate mitochondrial carriers, aralar and citrin: role in the heart malate–aspartate NADH shuttle. J Biol Chem 282:7098–7106
LaNoue KF, Meijer AJ, Brouwe A (1974) Evidence for electrogenic aspartate transport in rat liver mitochondria. Arch Biochem Biophys 161:544–550
Splawski I, Timothy KW, Sharpe LM, Decher N, Kumar P, Bloise R, Napolitano C, Schwartz PJ, Joseph RM, Condouris K, Tager-Flusberg H, Priori SG, Sanguinetti MC, Keating MT (2004) Ca(V)1.2 calcium channel dysfunction causes a multisystem disorder including arrhythmia and autism. Cell 119:19–31
Hope CI, Sharp DM, Hemara-Wahanui A, Sissingh JI, Lundon P, Mitchell EA, Maw MA, Clover GM (2005) Clinical manifestations of a unique X-linked retinal disorder in a large New Zealand family with a novel mutation in CACNA1F, the gene responsible for CSNB2. Clin Experiment Ophthalmol 33:129–136
Laumonnier F, Roger S, Guerin P, Molinari F, M’rad R, Cahard D, Belhadj A, Halayem M, Persico AM, Elia M, Romano V, Holbert S, Andres C, Chaabouni H, Colleaux L, Constant J, Le Guennec JY, Briault S (2006) Association of a functional deficit of the BKCa channel, a synaptic regulator of neuronal excitability, with autism and mental retardation. Am J Psychiatry 163:1622–1629
Palmieri L, Papaleo V, Porcelli V, Scarcia P, Gaita L, Sacco R, Hager J, Rousseau F, Curatolo P, Manzi B, Militerni R, Bravaccio C, Trillo S, Schneider C, Melmed R, Elia M, Lenti C, Saccani M, Pascucci T, Puglisi-Allegra S, Reichelt KL, Persico AM (2010) Altered calcium homeostasis in autism-spectrum disorders: evidence from biochemical and genetic studies of the mitochondrial aspartate/glutamate carrier AGC1. Mol Psychiatry 15:38–52
Lepagnol-Bestel A, Maussion G, Boda B, Cardona A, Iwayama Y, Delezoide A, Moalic J, Muller D, Dean B, Yoshikawa T, Gorwood P, Buxbaum J, Ramoz N, Simonneau M (2008) SLC25A12 expression is associated with neurite outgrowth and is upregulated in the prefrontal cortex of autistic subjects. Mol Psychiatry 13:385–397
Sakurai T, Ramoz N, Barreto M, Gazdoiu M, Takahashi N, Gertner M, Dorr N, Sosa M, Gasperi R, Perez G, Schmeidler J, Mitropoulou V, Le H, Lupu M, Hof P, Elder G, Buxbaum J (2010) Slc25a12 disruption alters myelination and neurofilaments: a model for a hypomyelination syndrome and childhood neurodevelopmental disorders. Biol Psychiatry 67:887–894
Zilbovicius M, Meresse I, Chabane N, Brunelle F, Samson Y, Boddaert N (2006) Autism, the superior temporal sulcus and social perception. Trends Neurosci 29:359–366
Ramoz N, Reichert JG, Smith CJ, Silverman JM, Bespalova IN, Davis KL, Buxbaum JD (2004) Linkage and association of the mitochondrial aspartate/glutamate carrier SLC25A12 gene with autism. Am J Psychiatry 161:662–669
Segurado R, Conroy J, Meally E, Fitzgerald M, Gill M, Gallagher L (2005) Confirmation of association between autism and the mitochondrial aspartate/glutamate carrier SLC25A12 gene on chromosome 2q31. Am J Psychiatry 162:2182–2184
Turunen JA, Rehnström K, Kilpinen H, Kuokkanen M, Kempas E, Ylisaukko-Oja T (2008) Mitochondrial aspartate/glutamate carrier SLC25A12 gene is associated with autism. Autism Res 1:189–192
Ramoz N, Cai G, Reichert JG, Silverman JM, Buxbaum JD (2008) An analysis of candidate autism loci on chromosome 2q24–q33: evidence for association to the STK39 gene. Am J Med Genet B Neuropsychiatr Genet 147:1152–1158
Silverman JM, Buxbaum JD, Ramoz N, Schmeidler J, Reichenberg A, Hollander E, Angelo G, Smith CJ, Kryzak LA (2008) Autism-related routines and rituals associated with a mitochondrial aspartate/glutamate carrier SLC25A12 polymorphism. Am J Med Genet B Neuropsychiatr Genet 147:408–410
Blasi F, Bacchelli E, Carone S, Toma C, Monaco AP, Bailey AJ, Maestrini E, International Molecular Genetic Study of Autism Consortium (IMGSAC) (2006) SLC25A12 and CMYA3 gene variants are not associated with autism in the IMGSAC multiplex family sample. Eur J Hum Genet 14:123–126
Rabionet R, McCauley JL, Jaworski JM, Ashley-Koch AE, Martin ER, Sutcliffe JS, Haines JL, DeLong GR, Abramson RK, Wright HH, Cuccaro ML, Gilbert JR, Pericak-Vance MA (2006) Lack of association between autism and SLC25A12. Am J Psychiatry 163:929–931
Correia C, Coutinho AM, Diogo L, Grazina M, Marques C, Miguel T, Ataíde A, Almeida J, Borges L, Oliveira C, Oliveira G, Vicente AM (2006) Brief report: High frequency of biochemical markers for mitochondrial dysfunction in autism: no association with the mitochondrial aspartate/glutamate carrier SLC25A12 gene. J Autism Dev Disord 36:1137–1140
Chien WH, Wu YY, Gau SS, Huang YS, Soong WT, Chiu YN, Chen CH (2010) Association study of the SLC25A12 gene and autism in Han Chinese in Taiwan. Prog Neuropsychopharmacol Biol Psychiatry 34:189–192
Pedersen P, Carafoli E (1987) Ion motive ATPases. I. Ubiquity, properties, and significance for cell function. Trends Biochem 14:146–150
Chicka MC, Strehler EE (2003) Alternative splicing of the first intracellular loop of plasma membrane Ca2+-ATPase isoform 2 alters its membrane targeting. J Biol Chem 278:18464–18470
Ficarella R, Di Leva F, Bortolozzi M, Ortolano S, Donaudy F, Petrillo M, Melchionda S, Lelli A, Domi T, Fedrizzi L, Lim D, Shull GE, Gasparini P, Brini M, Mammano F, Carafoli E (2007) A functional study of plasma-membrane calcium-pump isoform 2 mutants causing digenic deafness. Proc Natl Acad Sci USA 104:1516–1521
Bortolozzi M, Brini M, Parkinson N, Crispino G, Scimemi P, De Siati RD, Di Leva F, Parker A, Ortolano S, Arslan E, Brown SD, Carafoli E, Mammano F (2010) The novel PMCA2 pump mutation Tommy impairs cytosolic calcium clearance in hair cells and links to deafness in mice. J Biol Chem 285:37693–37703
Schultz JM, Yang Y, Caride AJ, Filoteo AG, Penheiter AR, Lagziel A, Morell RJ, Mohiddin SA, Fananapazir L, Madeo AC, Penniston JT, Griffith AJ (2005) Modification of human hearing loss by plasma-membrane calcium pump PMCA2. N Engl J Med 352:1557–1564
Rosenhall U, Nordin V, Brantberg K, Gillberg C (2003) Autism and auditory brain stem responses. Ear Hear 24:206–214
Rosenhall U, Nordin V, Sandstrom M, Ahlsen G, Gillberg C (1999) Autism and hearing loss. J Autism Dev Disord 29:349–357
Hu VW, Nguyen A, Kim KS, Steinberg ME, Sarachana T, Scully MA, Soldin SJ, Luu T, Lee NH (2009) Gene expression profiling of lymphoblasts from autistic and nonaffected sib pairs: altered pathways in neuronal development and steroid biosynthesis. PLoS One 4:e5775
Carayol J, Sacco R, Tores F, Rousseau F, Lewin P, Hager J, Persico AM (2011) Converging evidence for an association of ATP2B2 allelic variants with autism in males. Biol Psychiatry (in press)
Anney R, Klei L, Pinto D, Regan R, Conroy J, Magalhaes TR, Correia C, Abrahams BS, Sykes N, Pagnamenta AT, Almeida J, Bacchelli E, Bailey AJ, Baird G, Battaglia A, Berney T, Bolshakova N, Bölte S, Bolton PF, Bourgeron T, Brennan S, Brian J, Carson AR, Casallo G, Casey J, Chu SH, Cochrane L, Corsello C, Crawford EL, Crossett A, Dawson G, de Jonge M, Delorme R, Drmic I, Duketis E, Duque F, Estes A, Farrar P, Fernandez BA, Folstein SE, Fombonne E, Freitag CM, Gilbert J, Gillberg C, Glessner JT, Goldberg J, Green J, Guter SJ, Hakonarson H, Heron EA, Hill M, Holt R, Howe JL, Hughes G, Hus V, Igliozzi R, Kim C, Klauck SM, Kolevzon A, Korvatska O, Kustanovich V, Lajonchere CM, Lamb JA, Laskawiec M, Leboyer M, Le Couteur A, Leventhal BL, Lionel AC, Liu XQ, Lord C, Lotspeich L, Lund SC, Maestrini E, Mahoney W, Mantoulan C, Marshall CR, McConachie H, McDougle CJ, McGrath J, McMahon WM, Melhem NM, Merikangas A, Migita O, Minshew NJ, Mirza GK, Munson J, Nelson SF, Noakes C, Noor A, Nygren G, Oliveira G, Papanikolaou K, Parr JR, Parrini B, Paton T, Pickles A, Piven J, Posey DJ, Poustka A, Poustka F, Prasad A, Ragoussis J, Renshaw K, Rickaby J, Roberts W, Roeder K, Roge B, Rutter ML, Bierut LJ, Rice JP, Salt J, Sansom K, Sato D, Segurado R, Senman L, Shah N, Sheffield VC, Soorya L, Sousa I, Stoppioni V, Strawbridge C, Tancredi R, Tansey K, Thiruvahindrapduram B, Thompson AP, Thomson S, Tryfon A, Tsiantis J, Van Engeland H, Vincent JB, Volkmar F, Wallace S, Wang K, Wang Z, Wassink TH, Wing K, Wittemeyer K, Wood S, Yaspan BL, Zurawiecki D, Zwaigenbaum L, Betancur C, Buxbaum JD, Cantor RM, Cook EH, Coon H, Cuccaro ML, Gallagher L, Geschwind DH, Gill M, Haines JL, Miller J, Monaco AP, Nurnberger JI Jr, Paterson AD, Pericak-Vance MA, Schellenberg GD, Scherer SW, Sutcliffe JS, Szatmari P, Vicente AM, Vieland VJ, Wijsman EM, Devlin B, Ennis S, Hallmayer J (2010) A genome-wide scan for common alleles affecting risk for autism. Hum Mol Genet 19:4072–4082
Pessah IN, Cherednichenko G, Lein PJ (2010) Minding the calcium store: ryanodine receptor activation as a convergent mechanism of PCB toxicity. Pharmacol Ther 125:260–285
Vallipuram J, Grenville J, Crawford DA (2010) The E646D-ATP13A4 mutation associated with autism reveals a defect in calcium regulation. Cell Mol Neurobiol 30:233–346
Splawski I, Yoo DS, Stotz SC, Cherry A, Clapham DE, Keating MT (2006) CACNA1H mutations in autism spectrum disorders. J Biol Chem 281:22085–22091
Piton A, Gauthier J, Hamdan FF, Lafrenière RG, Yang Y, Henrion E, Laurent S, Noreau A, Thibodeau P, Karemera L, Spiegelman D, Kuku F, Duguay J, Destroismaisons L, Jolivet P, Côté M, Lachapelle K, Diallo O, Raymond A, Marineau C, Champagne N, Xiong L, Gaspar C, Rivière JB, Tarabeux J, Cossette P, Krebs MO, Rapoport JL, Addington A, Delisi LE, Mottron L, Joober R, Fombonne E, Drapeau P, Rouleau GA (2010) Systematic resequencing of X-chromosome synaptic genes in autism spectrum disorder and schizophrenia. Mol Psychiatry. doi:10.1038/mp.2010.54
Pavlowsky A, Gianfelice A, Pallotto M, Zanchi A, Vara H, Khelfaoui M, Valnegri P, Rezai X, Bassani S, Brambilla D, Kumpost J, Blahos J, Roux MJ, Humeau Y, Chelly J, Passafaro M, Giustetto M, Billuart P, Sala C (2010) A postsynaptic signaling pathway that may account for the cognitive defect due to IL1RAPL1 mutation. Curr Biol 20:103–115
Piton A, Michaud JL, Peng H, Aradhya S, Gauthier J, Mottron L, Champagne N, Lafrenière RG, Hamdan FF, S2D team, Joober R, Fombonne E, Marineau C, Cossette P, Dubé MP, Haghighi P, Drapeau P, Barker PA, Carbonetto S, Rouleau GA (2008) Mutations in the calcium-related gene IL1RAPL1 are associated with autism. Hum Mol Genet 17:3965–3974
Handley MT, Lian LY, Haynes LP, Burgoyne RD (2010) Structural and functional deficits in a neuronal calcium sensor-1 mutant identified in a case of autistic spectrum disorder. PLoS One 5:e10534
Sadakata T, Furuichi T (2009) Developmentally regulated Ca2+-dependent activator protein for secretion 2 (CAPS2) is involved in BDNF secretion and is associated with autism susceptibility. Cerebellum 8:312–322
Sadakata T, Washida M, Iwayama Y, Shoji S, Sato Y, Ohkura T, Katoh-Semba R, Nakajima M, Sekine Y, Tanaka M, Nakamura K, Iwata Y, Tsuchiya KJ, Mori N, Detera-Wadleigh SD, Ichikawa H, Itohara S, Yoshikawa T, Furuichi T (2007) Autistic-like phenotypes in Cadps2-knockout mice and aberrant CADPS2 splicing in autistic patients. J Clin Invest 117:931–943
Acknowledgments
We gratefully acknowledge the Maryland NICHD Brain & Tissue Bank for Developmental Disorders, the Harvard Brain Tissue Resource Center, and the Autism Tissue Program for providing the brain tissue samples assessed in our studies. The Persico Lab is supported by the Italian Ministry for University, Scientific Research and Technology (PRIN n. 2006058195 and 2008BACT54_002), the Italian Ministry of Health (RFPS-2007-5-640174), the Autism Speaks Foundation (Princeton, NJ), the Autism Research Institute (San Diego, CA), the Fondazione Gaetano e Mafalda Luce (Milan, Italy), and Autism Aids Onlus (Naples, Italy). The Palmieri lab is supported by the Italian Ministry for University, Scientific Research and Technology (PRIN n. 2008BACT54_002 and FIRB n. RBIN04PHZ7_001).
Conflict of Interest
We declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Napolioni, V., Persico, A.M., Porcelli, V. et al. The Mitochondrial Aspartate/Glutamate Carrier AGC1 and Calcium Homeostasis: Physiological Links and Abnormalities in Autism. Mol Neurobiol 44, 83–92 (2011). https://doi.org/10.1007/s12035-011-8192-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-011-8192-2