Abstract
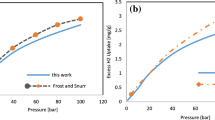
Analysis of methane (CH4) adsorption isotherms for metal–organic framework (MOF) adsorbents in the subcritical regions has been reported in this article. In this study, virgin MIL-101-(Cr) MOF, which is doped with alkali ions, are used to comprehend the influence of alkali dopants on CH4 sorption behaviour. MIL-101-(Cr) MOF and its derivatives with alkali ion dopants were prepared by the benign hydrothermal synthesis route, which was characterized by powder X-ray diffraction method. The equilibrium uptakes of CH4 under the subcritical condition were evaluated by fitting the isotherms with Langmuir, Toth and Dubinin–Astakohv adsorption models to determine the adsorption parameters. The adsorption parameters were correlated with the surface-induced heterogeneity, which can be attributed to the introduction of alkali dopants in the MOF structure. It is apparent from the analysis that the isosteric heat of adsorption diminishes with increasing alkali dopant size, while the induced surface structural heterogeneity increases with increasing alkali dopant size.










Similar content being viewed by others
References
Konstas K, Osl T, Yang Y, Batten M, Burke N J, Hill A R et al 2012 J. Mater. Chem. 22 698
Thamsiriroj T, Smyth H and Murphy J D 2011 Renew. Sustain. Energy. Rev. 15 642
He Y, Zhou W, Qian G and Chen B 2014 Chem. Soc. Rev. 43 5657
Waller M G, Williams E D, Matteson S W and Trabold T A 2014 Appl. Energy 127 55
Łaciak M, Sztekler K, Szurlej A and Włodek T 2019 IOP Conf. Ser. Earth Environ. Sci. 214 012138
Li J R, Kuppler R J and Zhou H C 2009 Chem. Soc. Rev. 38 1477
Menon V C and Komarneni S 1998 J. Porous Mater. 5 43
Ruthven D M 1984 Principles of adsorption and adsorption processes (New York: Wiley) p 82
Alcañiz-Monge J, Lozano-Castelló D, Cazorla-Amorós D and Linares-Solano A 2009 Micropor. Mesopor. Mater. 124 110
Peng Y, Krungleviciute V, Eryazici I, Hupp J T, Farha O K and Yildirim T 2013 J. Am. Chem. Soc. 135 11887
Duren T, Sarkisov L, Yaghi O M and Snurr R Q 2004 Langmuir 20 2683
Cabrera-Munguia D A, León-Campos M I, Claudio-Rizo J A, Solís-Casados D A, Flores-Guia T E and Cano-Salazar L F 2021 Bull. Mater. Sci. 44 245
Mason J A, Veenstra M and Long J R 2014 Chem. Sci. 5 22
Wegrzyn J and Gurevich M 1996 Appl. Energy 55 71
Hong D Y, Hwang Y K, Serre C, Ferey G and Chang J S 2009 Adv. Funct. Mater. 19 1537
Ferey G 2008 Chem. Soc. Rev. 37 191
Loiseaua T and Ferey G 2007 J. Fluor. Chem. 128 413
Kayal S, Sun B and Chakraborty A 2015 Energy 91 772
Aijaz A, Karkamkar A, Choi Y J, Tsumori N, Ronnebro E, Autrey T et al 2012 J. Am. Chem. Soc. 134 13929
Sun B, Chakraborty A, Ali S M and Kayal S 2016 Appl. Therm. Eng. 93 1175
Sircar S 2017 Adsorption 23 121
Loh W S, Rahman K A, Chakraborty A, Saha B B, Choo Y S, Khoo B C et al 2010 J. Chem. Eng. Data 55 2840
Rahman K A, Loh W S, Yanagi H, Chakraborty A, Saha B B, Chun W G et al 2010 J. Chem. Eng. Data 55 4961
He Y, Zhou W, Krishna R and Chen B 2012 Chem. Commun. 48 11813
Dubinin M M 1960 Chem. Rev. 60 235
Férey G, Mellot-Draznieks C, Serre C, Millange F, Dutour J, Surble S et al 2005 Science 309 2040
Abdulsalam J, Mulopo J, Bada S O and Oboirien B 2020 ACS Omega 5 32530
Aristov Y 2014 Appl. Therm. Eng. 72 166
Jagiello J 2004 Carbon 42 1227
Amankwah K A G and Schwarz J A 1995 Carbon 33 1313
Acknowledgements
This work was supported by the Centre for Sustainable Technology and Product Development.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Singh, A., Kayal, S. Investigation of methane adsorption onto metal–organic frameworks under subcritical condition employing adsorption isotherm models. Bull Mater Sci 45, 96 (2022). https://doi.org/10.1007/s12034-022-02685-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12034-022-02685-6