Abstract
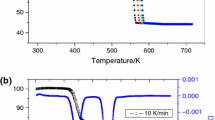
Cerium vanadate (CeVO4) and zirconium titanate (ZrTiO4) powders have been synthesized by the dicarboxylate coprecipitation method. The formation of precursors and mixed oxides was confirmed by their elemental and thermal analysis (thermogravimetric analysis–differential thermal analysis), infrared spectroscopy (Fourier transform infrared), X-ray fluorescence spectroscopy, energy-dispersive X-ray analysis and powder X-ray diffraction. X-ray analysis reveals the formation of a single-phasic CeVO4 with tetragonal structure and ZrTiO4 with orthorhombic structure. The morphological characteristics of these oxides were studied by field-emission scanning electron microscopy and a laser-based particle size analyzer. Different solid content and dispersants have been used to prepare various suspensions of these oxides in distilled water and ethanol. The rheological flow behaviour of the suspensions has been studied by measuring their viscosity against the increasing shear rate. The power-law model is applied to calculate consistency coefficient (k) and flow behaviour index (n). Minimum viscosities have been observed at pH = 10.6 for CeVO4–water–Darvan system and at pH = 10.5 for ZrTiO4–water–polyethylene imine system.
Graphic abstract












Similar content being viewed by others
References
Jindal R, Sinha M M and Gupta H C 2013 Spectrochim. Acta A Mol. Biomol. 113 286
Hou J, Huang H, Han Z and Pan H 2013 RSC Adv. 6 14552
Jin R, Liu C, Sun L, Zhang Z and Chen G 2016 ChemElectroChem. 3 644
Yang S L and Wu J M 1991 J. Mater. Sci. 26 631
Jain M K, Bhatnagar M C and Sharma G L 1999 Sens. Actuators B Chem. 55 180
Cosentino I C, Muccillo E N S and Muccillo R 2003 Sens. Actuators B Chem. 96 677
Wakino K, Minai K and Tamura H 1984 J. Am. Ceram. Soc. 67 278
Leoni M, Viviani M, Battilana G, Fiorello A M and Viticoli M 2001 J. Eur. Ceram. Soc. 21 1739
Daly F P, Ando H, Schmitt J L and Sturm E A 1987 J. Catal. 108 401
Reddy B M, Sreekanth P M, Yamada Y, Xu Q and Kobayashi T 2002 Appl. Catal. A Gen. 228 269
Chang D A, Lin P and Tseng T Y 1995 J. Appl. Phys. 77 4445
Denisova L T, Chumilina L G, Kargin Y F and Denisov V M 2016 Inorg. Mater. 52 44
Opara Krasovec U, Orela B, Surcaa A, Bukovecb N and Reisfeldc R 1999 Solid State Ion. 118 195
Liu F, Shao X, Yin Y, Zhao L, Sun Q, Shao Z et al 2011 J. Rare Earths 29 97
Wang H, Meng Y Q and Yan H 2004 Inorg. Chem. Commun. 7 553
Xie B, Lu G, Dai Q, Wang Y, Guo Y and Guo Y 2001 J. Clust. Sci. 22 555
Othman I, Hisham Zain J, Abu Haija M and Banat F 2020 Appl. Catal. B Environ. 266 118601
Nikumbh A K and Adhyapak P V 2010 Powder Technol. 202 14
El-Sayed A M 2002 Ceram. Int. 28 363
John R and Rajakumari R 2012 Nano-Micro Lett. 4 65
Nikumbh A K, Nagawade A V, Gugale G S, Chaskar M G and Bakare P P 2002 J. Mater. Sci. 37 637
McGeary R K 1961 J. Am. Ceram. Soc. 44 513
Vecer M and Pospisil J 2012 Proc. Eng. 42 1720
Laarz E, Zhmud B V and Bergström L 2000 J. Am. Ceram. Soc. 83 2394
Tarì G, Ferreira J M F and Lyckfeldt O 1998 J. Eur. Ceram. Soc. 18 479
Sigmund W M, Bell N S and Bergström L 2000 J. Am. Ceram. Soc. 83 1557
Bergström L 1998 Colloids Surf. A Physicochem. Eng. Asp. 133 151
Hoebbel D, Reinert T, Schmidt H and Arpac E 1997 J. Sol-Gel. Sci. Technol. 10 115
Zhang J X, Jiang D L, Tan S H, Gui L H and Ruan M L 2001 J. Am. Ceram. Soc. 84 2537
Acknowledgement
We would like to acknowledge Mr Yogesh Yewale from Ahmednagar College, Ahmednagar, and Dr J K Khedkar, from Shri Anand College, Pathardi, for their suggestions and timely help.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nagawade, A.V., Nikumbh, A.K., Karale, N.J. et al. An investigation of the formation, the particulate properties and the rheological behaviour of cerium vanadate and zirconium titanate prepared by dicarboxylate coprecipitation method. Bull Mater Sci 45, 92 (2022). https://doi.org/10.1007/s12034-021-02641-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12034-021-02641-w