Abstract
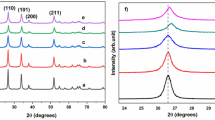
SnO2 nanoparticles are synthesized via sol–gel method in the presence of two surfactants isopropyl alcohol (IPA) and lauryl alcohol (LA). The synthesized nanoparticles are characterized for the microstructural features by X-ray powder diffraction (XRD) and field-emission scanning electron micrograph. To resolve the presence of defect-related oxygen vacancies and trapped states, optical studies such as UV–visible absorbance and Raman spectra have been carried out. The charge transport in the material is analysed by studying the AC electrical conduction. The deposited tetragonal rutile-phased SnO2 nanoparticles are benefited by morphological modifications along with crystallite size reduction (from 21.5 to 15.1 nm) and an increase in dislocation density on changeover from LA to IPA in the precursor. Also, a preferential orientation of (112) plane is observed for the LA-assisted sample. The bandgap of the particles prepared via LA addition is found to be considerably larger (3.71 eV) than that of IPA-assisted sample (3.44 eV). A detailed study of Raman spectra elucidates the presence of defects. The AC conductivity analysis reveals that the mobility of charge carriers is higher in LA-assisted sample, which substantiates the findings from microstructural and optical studies.









Similar content being viewed by others
References
Yang Y, Wang Y and Yin S 2017 Appl. Surf. Sci. 420 399
Zhao Q, Ma L, Zhang Q, Wang C and Xu X 2015 J. Nanomater. Article ID 850147 https://doi.org/10.1155/2015/850147
Chen J S and Lou X W 2013 Small 11 1877
Pan J, Shen H and Mathur S 2012 J. Nanotechnol. Article ID 917320 https://doi.org/10.1155/2012/917320
Suthakaran S, Dhanapandian S, Krishnakumar N and Ponpandian N 2019 Mater. Res. Express 6 0850i3
Balakrishnan K and Murugasean N 2021 Int. J. Nano Dimens. 12 76
Majumdar S and Devi P S 2010 AIP Conf. Proc. p 1
Kumar P, Khadtare S, Park J and Yadav B C 2020 Mater. Lett. 278 128451
Bhagwat A D, Sawant S S, Ankamwar B G and Mahajan C M 2015 J. Nano Electron. Phys. 7 04037
Aziz M, Abbas S S and Baharom W R W 2013 Mater. Lett. 91 31
Kumari K P, Thomas B, Deepa S and Benoy S 2018 J. Mater. Sci: Mater. Electron. 29 13087
Maestro A, Santini E, Zabiegaj D, Llamas S, Ravera F, Liggieri L et al 2015 Ad. Condens. Matter Phys. Article ID 917516 doi:https://doi.org/10.1155/2015/917516
Patil G E, Kajale D D, Gaikwad V B and Jain G H 2012 Int. Nano Lett. 2 1
Winchell A N and Winchell H 1964 The microscopical characters of artificial inorganic solid substances: optical properties of artificial minerals (Academic Press)
Suryanarayana C and Norton M G 2013 X-ray diffraction: a practical approach (Springer Science & Business Media)
Cullity B D 1957 Sci. Am. 196 103
Bahadar K S, Faisal M, Rahman M M and Jamal A 2011 Sci. Total Environ. 409 2987
Devesa S, Rooney A P, Graça M P, Cooper D and Costa L C 2021 Mater. Sci. Eng.: B 263 114830
Nasser S A, Afify H H, El-Hakim S A and Zayed M K 1998 Thin Solid Films 315 327
Swarnkar R K, Singh S C and Gopal R 2011 Bull. Mater. Sci. 34 1363
Deepa S, Kumari K P and Thomas B 2017 Ceram. Int. 43 17128
Zhou J X, Zhang M S, Hong J M and Yin Z 2006 Solid State Commun. 138 242
Diéguez A, Romano-Rodríguez A, Vila A and Morante J R 2001 J. Appl. Phys. 90 1550
Chen Y J, Nie L, Xue X Y, Wang Y G and Wang T H 2006 Appl. Phys. Lett. 88 083105
Thangadurai P, Chandra B A, Ramasamy S, Kesavamoorthy R and Ravindran T R 2005 J. Phys. Chem. Solids 66 1621
Sahay P P, Mishra R K, Pandey S N, Jha S and Shamsuddin M 2012 Ceram. Int. 38 1281
Bredar A R, Chown A L, Burton A R and Farnum B H 2020 ACS Appl. Energy Mater. 3 66
Kungumadevi L, Sathyamoorthy R and Subbarayan A 2010 Solid State Electron. 54 58
Ghosh M and Rao C N R 2004 Chem. Phys. Lett. 393 493
Lanje A S, Sharma S J and Pode R B 2010 Arch. Phys. Res. 1 49
Aal A A 2006 Egypt J. Solids 29 303
Acknowledgement
This study was supported by RUSA (File No. 009/2019-20/RUSA MRP (S)/MAC).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Deepa, S., Philip, A.M., George, A.S. et al. Microstructural, optical and dielectric variations in SnO2 nanoparticles synthesized via surfactant-assisted sol–gel route. Bull Mater Sci 44, 283 (2021). https://doi.org/10.1007/s12034-021-02567-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12034-021-02567-3