Abstract
COVID-19 pandemic started more than a year ago and has infected more than 115 million of people from ~210 countries and >2.5 million of deaths worldwide being reported without any commercial and effective treatment or vaccine being yet released. However, recent studies on nanomaterials such as fullerenes, carbon nanotubes and graphene showed that they possess anti-inflammatory, antiviral, anti-oxidant and anti-HIV properties. Herein, the interactions which established between the fullerenes Cm (m = 48, 60, 70, 80, 84 and 86) and the spike protein (SP) of SARS-CoV-2 and the human ACE2 receptor have been investigated based on the density functional theory (DFT) method with the CAM-B3LYP functional and the 6-31G* basis. The results of this study show that C48 exhibited as potential inhibitor of SARS-CoV-2. Because of the presence of heteroatoms on the surface of fullerenes which systematically reduce energy gaps, which in turn increase their reactivities. The oxygen adsorbed by fullerenes increases the number of non-covalent contacts and involves a large number of hydrogen bonds, while decreasing the binding energies. Thus, the hACE2-SP-4O2@C60 complex is strongly recommended for inhibiting SARS-CoV-2 in the final phase of contamination.
Graphic abstract
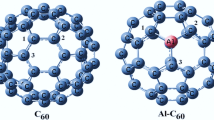
Stabilizing interactions between fullerenes and the spike protein of SARS-CoV-2.

Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
C60 is the first member of the fullerene family to be synthesized. It contains only carbon atoms with 60π electrons which are not completely delocalized due to its crystallographic structure [1]. Indeed, the C60 has a truncated icosahedrons structure with 32 faces, including 20 hexagons and 12 pentagons, each of these being surrounded by six hexagons.
The crystallographic structure reveals that the C–C bond lengths in the pentagons are identical and equal to 1.45 Å which is characteristic of a single Csp3–Csp3 type, while in the hexagons, the bond lengths are alternately of 1.45 Å (single bond) which are common to a pentagon and a hexagon and 1.38 Å (double bonds) which are common to two hexagons. The localization of π electrons would make the carbon atoms in the C60 non-identical, leading to a different possibility of interactions. This could suggest that Cm (m = 48, 60, 70, 80, 84 and 86) would be a potential candidate for inhibition of SARS-CoV-2.
It has been more than a year since the COVID-19 [2] pandemic has spread in the world with an upsurge in the number of infected people that exceeds 115 million worldwide with >2.5 million of deaths without any treatment or effective vaccine being proposed until now. To propose those which inhibit the SARS-CoV-2 and based on the databases of molecules, several molecular docking studies have been carried out. Nanomaterials, such as fullerenes, nanotubes and graphene have been shown to interact strongly with peptide and protein assemblies [3,4] and have antiviral [5,6,7,8,9,10,11], anti-oxidant, anti-inflammatory [12] and anti-HIV [9] properties. The use of fullerenes in medicine is increasingly explored even if several studies warn of potential damage caused to the living organisms [13,14] and even in-vivo studies have shown that the C60 and its derivatives are not toxic [15,16]. It would be necessary to understand in advance, the interactions that are established between fullerenes and the spike protein (SP) as well as with the human angiotensin-converting enzyme 2 (hACE2). On the other hand, it has been showed that in agreement with the experience that C60 binds strongly with Aβ fibrils and the binding energy decreases with increase in the size of fullerenes [17]. In addition, one of the main characteristics of the C60 is its ability to capture free radicals that protects biological systems from cell destruction [18,19]. The presence of several double bonds is responsible for scavenging free radicals [20] and probably for establishing strong non-covalent interactions between fullerenes and the various residues of the spike protein and the hACE2 receptor. Moreover, it has been showed that the hydroxychloroquine [21] and adsorption of chloroquine on C60 fullerenes and the doped one with B, Al and Si are thermodynamically stable [22] and are potential carriers of drugs [23,24,25].
For all these reasons and in the absence of treatment for the COVID-19 pandemic, the fullerenes can be an approach to be exploited [17], which means it is interesting to study the interactions between the nanomaterials in the different sites of the contact area (figure 1) between SARS-CoV-2 and the hACE2 receptor. Furthermore, a recent experimental [26] study showed that the lipid layer present in the virus of COVID-19 can be deactivated by the nanocarbon-coated surfaces, such as fullerenes. Other studies showed anti-cancer effects of fullerene derivatives [27,28,29]. Herein, our work reports the exploitation of the electronic and structural characteristics of fullerenes and the examination of their interactions with the spike protein and hACE2 receptor.
For this purpose, we use the density functional theory (DFT) method with the CAM-B3LYP functional and the 6-31G* basis sets for the determination of most stable structures of the different ligands (Cm, m = 48, 60, 70, 80, 84 and 86) and fullerenes adsorbing oxygen molecules Cm@nO2 [30] without any symmetry constraint. Chimera 1.14 [31] software is used to perform molecular docking. We use the cleaned PDB files (6LZG and 6MOJ) (figure 1), from the databank from which we extracted the structures of the spike protein and hACE2 receptor.
2 Computational details
Fullerenes C48(D6d), C60(Ih), C70(D5h) and C80(C2v) have been optimized without any symmetry constraint at the DFT levels using the CAM-B3LYP functional [32,33,34] and the split valance polarized basis set 6-31G* implemented in Gaussian 03 [35]. The PDB file of SARS-CoV-2 spike protein (SP) RBD-hACE2 complex (PDB ID 6LZG or 6MOJ) was obtained from the structural bioinformatics (RCSB) protein data bank (PDB) (http://www.rcsb.org/structure/6LZG). We have used the UCSF Chimera 1.14 [31] to highlight the structure of the ligand and/or protein-complex structure, to perform the various functions associated with ligand and protein preparations and acting as an interface to enable molecular docking calculations using locally hosted AutoDock Vina software [36]. Prior to molecular docking, the hACE2 protein (part A) and S-protein (part B) in 6LZG PDB files were deleted from the PDB file of the complex, respectively, to study the S-protein-fullerene (SP@Cm) and hACE2-fullerene (hACE2@Cm) complexes. In addition, all non-standard residues including that of water were also removed. The structure of each fullerene ligand has been incorporated into UCSF Chimera [31] using the optimized structure at the CAM-B3LYP/6-31G* levels, which also have been imported in the PDB file format obtained from Gaussian 03 software calculation at B3LYP/6-31G* levels [35] and visualized by GaussView 5.0.9 package [37]. The PDBQT files of the S-protein RBD and fullerene ligands were generated after adding all hydrogen and charges to each structure. The S-protein and hACE2 docking search box were assigned by varying the receptor search volume in terms of size and centre to cover the contact area previously observed between the S-protein RBD and hACE2 receptor. The number of binding modes was fixed to 10 with exhaustiveness of search set to eight. The maximum energy difference was fixed up to 3 kcal mol-1. The obtained molecular docking results were then aligned with the PDBQT files of the S-protein, hACE2 and S-protein RBD-hACE2 complex to emphasize the interaction type of docked complexes in the S-protein, hACE2 and S-protein-hACE2 binding interface, respectively. The AMBER ff14SB force field was used [38].
3 Results and discussion
3.1 Structural studies of different ligands
The results giving the 3D optimized geometries of different fullerenes (Cm, figure 2a) and different fullerenes adsorbed by nO2 molecules nO2@Cm (figure 2b) are illustrated in figure 2. The calculations, for the energy minima, lead to normal modes of vibration which are all real. Overall, when fullerene size increases, the energy gap decreases, so the reactivity increases.
(a) Optimized structure of C48(D6d), C60(Ih), C70(D5h), C80(C2v) and (b) C60, C60@O2, C60@2O2 and C60@4O2 obtained at the CAM-B3LYP/6-31G* levels with ionization potential I, electron affinity A, energy gap, chemical potential μ, chemical hardness η, electrophilicity ω, nucleophilicity ν in eV and molecular volume in Å3 evaluated by Chimera software.
The studies of the physicochemical descriptors [39]:

where χM is the Mulliken electronegativity, I the ionization potential, A the electron affinity, μ the chemical potential, η the chemical hardness, ω the electrophylicity and ν the nucleophilicity, which are calculated at the CAM-B3LYP/6-31G* level with the Koopmans approximation [40], show that the C60@nO2 complexes with fullerene are more reactive than the C60. In fact, energy gap, electrophilicity ω, e chemical potential μ, electronegativity χ (−μ) and nucleophilicity ν increase in absolute values in all cases when we go from C60 to complexes.
The IR spectra given in figure 3, show the normal mode of vibration calculated at the CAM-B3LYP/6-31G* level where the IR peak half-width at half-height is multiplied by 40 cm−1.
From the IR spectra (figure 3), the band appearing around 588 cm−1 for C60 disappears as the number of adsorbed oxygen molecules increase; it completely disappears for the C60@4O2 complex. It is the same for the bands that appear between 1214 and 1460 cm−1.
A new band, large and intense, characteristic of oxygenated compound: C–O elongations symmetrical (\( \nu _{{{\text{C}} - {\text{O}}}}^{{{\text{Sym}}}} \)) at 1070 cm−1 and asymmetric (\( \nu _{{{\text{C}} - {\text{O}}}}^{{{\text{aSym}}}} \)) at 1170 cm−1 appear for the fullerene complexes. The vibration spectra of all other Cm@nO2 complexes with m = 48, 70, and 80 with n = 1, 2 and 4 lead to the same conclusions.
The calculated area under the curve (AUC) of the characteristic peak at 1000–1200 cm−1 of adsorbed, as function of the number of adsorbed oxygen molecules nO2, is shown in figure 4. We note that the surface increases linearly with the number nO2. This result confirms the fixation and the quantities of oxygen adsorbed.
From the frontier molecular orbital (FMO), a clear reduction in the energy gap (figure 2b) is observed which is corresponding to a greater reactivity. The shape of the FMOs, given in figure 5, shows a different distribution of the C60@nO2 complexes.
3.2 Docking results
3.2a Fullerene ligand: Taking into account the contact interface between the spike protein of SARS-CoV-2 and the hACE2 (figure 1), we have opted for all the docking calculations with the size of the box modelling in this interface, such as special parameters: centre: −33.57; 32.45; 1.938 and x = 22.0; y = 45.0 and z = 20.0.
The results of the SP@Cm and hACE2@Cm complexes, shown in table 1, highlight the following:
(i) The interaction energies are non-covalent types.
(ii) Overall, the binding energy decreases with increase in the sizes of the fullerene. This trend is not observed for C48 and C84, this exception is due to the flattened shape of the structure that promotes better contacts with the target as discussed further.
The same findings also apply for complexes hACE2@Cm (m = 48, 60, 70, 80, 84 and 86) (table 1). Indeed, when the shape of the fullerene is oval or flattened, the corresponding binding energy is lower with more non-covalent contacts between the spike protein and hACE2 targets and ligand.
From the different interactions established (table 2), it appears that:
(i) The residues of the spike protein involved are identical from fullerene C48 to C86. C48 established 48 interactions due to its flattened shape with a binding energy of −8.0 kcal mol−1 (figure 6a). All 10 stable structures are located at down position of the spike protein (figure 6b). However, for C86 form 34 contacts with a binding energy of −8.6 kcal mol−1. The order of magnitude of the binding energies is a neighbour, but C48 would link better to the spike protein.
(ii) The residues involved in hACE2@Cm are also identical in all cases implicating the same conclusion as above. Even if the lowest binding energy corresponds to the hACE2@C86 complex (−9.4 kcal mol−1, table 1), the advantage of the contacts is in favour of the hACE2@complex C48 (binding energy of −8.4 kcal mol−1, figure 7a and b) with 53 contacts which correspond to the following residues: THR27(13), PHE28(9), LYS3(13), LEU79(12) and TYR83(6) (in brackets the number of contacts per residue).
(iii) For the complexes hACE2@SP@Cm type (m = 48, 60, 70, 80, 84 and 86), the advantage is also attributed for the smaller fullerene sizes (C48) with a binding energy of −8.5 kcal mol−1 and 86 interactions distributed as follows: ARG403(18, SP), LYS417(17, SP), PRO389(17, hACE2), ASN33(9, hACE2), ASP30(4, hACE2), HIS34(10, SP), TYR505(2, SP), ARG393(5, SP), GLN388(2, hACE2) and ALA387(2, hACE2); including 52 contacts with the spike protein and 34 with the hACE2 receptor. While for the other fullerenes, considering their molecular volume (figure 2), their binding energies and the number of contacts they established (tables 1 and 2), they cannot be inhibitors of SARS-CoV-2 because they linked outside of the contact area between the spike protein and the hACE2 receptor (figure 8).
The different interactions of both stable conformations are represented in figure 7a and b.
Figure 9 shows the hydrophobic contacts between SER494, GLY496, TYR449, TYR505 and GLN498 residues of the spike protein and the corresponding atoms of the ligand obtained with the LigPlot+ v.2.2 multiple ligand–protein interaction diagrams of drug discovery [41].
Hydrophobic contacts between the residues of the spike protein (table 2) and corresponding atoms of the C48 ligand.
Figure 10 shows, in 2D, the 64 hydrophobic contacts between GLN96, LYS26, VAL93, ASP30, ASN33, THR92 and PRO389 residues of the ACE2 receptor and corresponding atoms of ligand.
Hydrophobic contacts between the residues of the ACE2 receptor (table 2) and corresponding atoms of the C48 ligand.
3.2b Complex Cm@nO2 ligand: SARS-CoV-2 is found to cause several and very different symptoms, such as hypoxemia (lack of oxygen). Once the cell is infected with SARS-CoV-2, it depletes the cell in oxygen. It is known that fullerenes adsorb oxygen molecules and the presence of heteroatoms on them systematically reduces the energy gap, this means that the exchange of electrons is greater and become most reactive compared to pure fullerenes [42,43]. The presence of oxygen atoms on the surface of fullerenes will promote the formation of hydrogen bonds with various targets (spike protein and hACE2), which will strengthen the binding of modified fullerenes to these targets and thus inhibit the penetration of SARS-CoV-2 inside the cell. For these reasons, we have optimized at the CAM-B3LYP/6-31G* level, without symmetry constraints for the complexes: Cm@nO2 (n = 1, 2 and 4 and m = 48, 60 and 80) (table 3). PDB files were created using GaussView 5.0.9 and transferred to Chimera 1.14 software where molecular docking was performed by studying their interactions with the spike protein and hACE2.
The adsorption of oxygen by the various fullerenes decreases the values of the binding energy (see figure 2b) and increases the number of contacts by including stabilizing interactions through the formation of hydrogen bonds, e.g., the SP-C48@2O2 has a binding energy of −9.1 kcal mol−1 with 43 contacts whose five-hydrogen bonding, compared to that of SP-C48 complex which is of −8.0 kcal mol−1 with 48 non-covalent contacts without any hydrogen bond. On the other side, the SP-C80 complex has a binding energy of −8.5 kcal mol−1 with 32 non-covalent contacts, while that of the SP-C80@4O2 complex is −10.7 kcal mol−1 with 42 contacts including seven hydrogen bonds, i.e., a difference of 2.2 kcal mol−1 is in favour of the stability of the SP-C80@4O2 complex, especially, since all the contacts are established inside the spike protein–hACE2 interface. In all cases, the adsorption of oxygen on the fullerenes promotes the binding of the ligand to the spike protein and to the hACE2 receptor with a difference in stabilizing binding energy of around 1.2 kcal mol−1 with a great number of contacts including hydrogen bonds. The most favourable case is the hACE2-SP-C60@4O2 complex with a binding energy of −13.2 kcal mol−1 compared to the hACE2-SP-C60 complex with a binding energy of −5.1 kcal mol−1. Once the spike protein is attached to the hACE2 receptor, that is to say, in the final phase of contamination, the hACE2-SP-C60@4O2 complex is strongly recommended to inhibit the action of SARS-CoV-2; the difference in binding energy is of −8.1 kcal mol−1 with 18 hydrogen bonds (with the 10 stable structures) that form with the complex where four oxygen molecules are adsorbed on the C60.
For example, for the most stable conformation of C48@4O2, three hydrogen bonds are formed, two between ARG403 (2.79 and 1.81 Å) and oxygen O6 (figure 6a) where the third did not appear (complexity of 2D representation), is established between the TYR505 and O7 (figure 11a) which is 2.51 Å (figure 11b).
3.2c H2O solvent effect: Water as a solvent is a capital importance, if we research stabilizing interaction between the different targets and the adsorbed fullerenes. The study of solvent effect has not been investigated in this work because of two considerations:
(i) Adsorbed fullerenes can be used as a layer covering protective masks to trap SARS-CoV-2 because of this solid physical state. So, considering the effect of solvent is unnecessary in this case.
(ii) If the adsorbed fullerenes are administrated, the effect of solvent (water) increases the contact area between the targets and the ligands, and therefore, the stabilizing interactions would be greater. In fact, the successive layers of water molecules which are linked to the adsorbed fullerenes would be formed by hydrogen bonds as shown in figure 12. Therefore, the contact area is identical without or with solvent.
4 Conclusion
From this study, it emerges that fullerenes are potential inhibitors of SARS-CoV-2 because of the number of interactions which are established with the spike protein and the hACE2 receptor, respectively. C48 presents as the best asset due to its flattened shape which allows it to have a greater inhibitory surface at the contact interface between the spike protein and the hACE2 receptor. The adsorption of oxygen molecules on various fullerenes increases the non-covalent contacts and creates a new interaction with hydrogen bonds which stabilize the various complexes, such as SP-Cm@nO2, ACE2-Cm@nO2 and ACE2-SP-Cm@nO2. In the final phase of contamination, the hACE2-SP-C60@4O2 complex is strongly recommended to inhibit the action of SARS-CoV-2 because of its binding energy and the large number of hydrogen bond interactions.
References
Kroto H W, Heath J R, O’Brien S C, Curl R F and Smalley R E 1985 Nature 318 162
Gupta V 2020 Lett. Appl. NanoBioScience 9 1083
Sivasankarapillai V S, Pillai A M, Rahdar A, Sobha A P, Sachi Das S, Mitropoulos A C et al 2020 Nanomaterials 10 852
Li C and Mezzenga R 2013 Nanoscale 5 6207
El Haes H, Saleh N A, Omar A and Ibrahim M 2014 J. Comput. Theor. Nanosci. 11 2136
Hammed A J, Ibrahim M and El Haes H 2007 J. Mol. Struct. Theochem. 809 131
Saleh N A, El Haes H, Osman O, Mahmoud A A and Ibrahim M 2015 Open Spectrosc. J. 9 1
Liliana I, Nosik N N and Kondrashina N G 2016 Int. J. Infect. Dis. 53 82
Nosik D N, Lialina I K, Kalnina L B, Lobach O A, Chataeva M S and Rasnetsov L D 2009 Vopr. Virusol. 54 41
Innocenzi P and Stagi L 2020 Chem. Sci. 11 6606
Suku A S, Jayakumar A and Valappil M P 2020 Lett. Appl. NanoBioScience 9 1637
Klimova R, Andreev S, Momotyuk E, Demidova N, Federova N, Chernoryzh Y et al 2020 Fuller. Nanotub. Carbon Nanostruct. 28 487
Kayat J, Gajbhiye V, Tekade R K and Jain N K 2011 Nanomedicine 7 40
Yang S T, Liu Y, Wang Y W and Cao A 2013 Nano. Micro Small 9 1635
Moussa F, Trivin F, Ceolin R, Hadchouel M, Sizaret P Y, Greugny V et al 1996 J. Fuller. Sci. Technol. 4 21
Bobylev A G, Okuneva A D, Bobyleva L G, Fadeeva I S, Fadeev R S, Salmov N N et al 2014 Biofizika 59 843
Huy P D Q and Li M S 2014 Phys. Chem. Chem. Phys. 16 20030
Bakry R, Vallant R M, Najam-Ul-Haq M, Rainer M, Szabo Z, Huck C W et al 2007 Int. J. Nanomed. 2 639
Sun T and Xu Z 2006 Bioorg. Med. Chem. Lett. 16 3731
Goodarzi S, Da Ros T, Conde J, Sefat F and Mozafari M 2017 Mater. Today 20 460
Dubey D 2020 Lett. Appl. NanoBioScience 9 1705
BagheriNovir S and Reza Aram M 2020 Chem. Phys. Lett. 757 137869
Kuznietsova H, Dziubenko N, Herheliuk T, Prylutskyy Y, Tauscher E, Ritter U et al 2020 Pharmaceutics 12 794
Nalepa P, Gawecki R, Szewczyk G, Balin K, Dulski M, Sajewicz M et al 2020 Cancer Nano. 11 2
Huang H J, Kraevaya O A, Voronov I, Troshin P A and Hsu S H 2020 Int. J. Nanomed. 15 20486
Siddiquie R Y, Agrawal A and Joshi S 2020 Trans. Indian Natl. Acad. Eng. 5 343
Tatyanenko L V, Pokidova O V, Goryachev N S, Kraevaya O A, Khakina E A, Yu Belik A et al 2020 Bull. Exp. Biol. Med. 69 89
Xu H, Tu X, Fan G, Wang Q, Wang X and Chu X 2020 J. Mol. Liq. 318 114315
Bagheri Novir S and Reza Aram M 2020 Chem. Phys. Lett. 757 137869
Tabari L and Farmanzadeh D 2019 Appl. Surf. Sci. 479 569
Pettersen E F, Goddard T D, Huang C C, Couch G S, Greenblatt D M, Meng E C et al 2004 J. Comput. Chem. 25 1605
Becke A D J 1993 Chem. Phys. 98 5648
Lee C, Yang W and Parr R G 1988 Phys. Rev. B 37 785
Stephens P J, Devlin F J, Chabalowski C F and Frisch M J 1994 J. Phys. Chem. 98 11623
Frisch M J, Trucks G W, Schlegel H B, Scuseria G E, Robb M A, Cheeseman J R et al 2004 Gaussian 03, Revision C.02. Gaussian Inc, Wallingford, CT
Trott O and Olson A J 2010 J. Comput. Chem. 31 455
Nielsen A B and Holder A J 2009 Gauss View 5.0, User’s Reference. Gaussian Inc., Pittsburgh
Maier J A, Martinez C, Kasavajhala K, Wickstrom L, Hauser K and Simmerling C 2015 J. Chem. Theory Comput. 11 3696
Makov G 1995 J. Phys. Chem. 99 9337
Koopmans T 1934 Physica 1 104
Laskowski R A and Swindells M B 2011 J. Chem. Inf. Model. 51 2778
Wang Y, Jiao M, Song W and Wu Z 2017 Carbon 114 393
Gao F, Zhao G L, Yang S and Spivey J J 2013 J. Am. Chem. Soc. 135 3315
Acknowledgements
This study was supported by DGRSDT/ATRST (project-COVID-19/2020) and ‘Centre de Recherche en Analyses Physico-Chimiques (CRAPC)’ from Algeria.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Brahimi, M., SELLAM, D., Bouchoucha, A. et al. In-silico modelling of fullerene and fullerene adsorbed by nO2 molecules (n(O2)@Cm with n = 1, 2, 4 and m = 48 and 60) as potential SARS-CoV-2 inhibitors. Bull Mater Sci 44, 220 (2021). https://doi.org/10.1007/s12034-021-02505-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12034-021-02505-3