Abstract
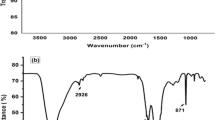
In-situ electrochemical deposition of cobalt hexacyanoferrate (CoHCF) on graphene oxide (GO) and its application for the electrocatalytic hydrazine determination in real samples are described in this research study. Co2+ is immobilized on GO and the resulting material, GO-Co2+ is coated on the surface of glassy carbon (GC) electrode. The fabricated electrode (GC/GO-Co2+) is subjected to a continuous potential cycling in the range of 0.0–1.0 V which results in the formation of a thin CoHCF film on the surface of GO coated on the GC electrode (abbreviated as GC/GO-CoHCF). The synthesized GO-CoHCF composite material is characterized by Fourier transform infrared and scanning electron microscopy. GC/GO-CoHCF electrode electrocatalytically oxidizes hydrazine at low overpotential (0.63 V) and this phenomenon is subsequently utilized for the sensitive determination of hydrazine in aqueous solutions. It exhibits a wide linear calibration range (0.1–400 µM), high sensitivity (0.93 µA µM−1 cm−2) and low limit of detection (17.5 nM) for the determination of hydrazine. Further, this electrode is employed for hydrazine determination in real samples.
Graphic abstract







Similar content being viewed by others
References
Georgakilas V, Otyepka M, Bourlinos A B, Chandra V, Kim N, Kemp K C et al 2012 Chem. Rev. 112 6156
Jahan M, Bao Q and Loh K P 2012 J. Am. Chem. Soc. 134 6707
Zhang H, Li Z-F, Snyder A, Xie J and Stanciu L A 2014 Anal. Chim. Acta 827 86
Dreyer D R, Park S, Bielawski C W and Ruoff R S 2010 Chem. Soc. Rev. 39 228
Kuila T, Bose S, Mishra A K, Khanra P, Kim N H and Lee J H 2012 Prog. Mater. Sci. 57 1061
Shi J, Zhang H, Snyder A, Wang M-X, Xie J, Porterfield D M et al 2012 Biosens. Bioelectron. 38 314
Marcano D C, Kosynkin D V, Berlin J M, Sinitskii A, Sun Z, Slesarev A et al 2010 ACS Nano 4 4806
Castro S S L, Mortimer R J, de Oliveira M F and Stradiotto N R 2008 Sensors 8 1950
Li X, Chen Z, Zhong Y, Yang F, Pan J and Liang Y 2012 Anal. Chim. Acta 710 118
Florescu M, Barsan M, Pauliukaite R and Brett C M A 2007 Electroanalysis 19 220
Yang M, Jiang J, Yang Y, Chen X, Shen G and Yu R 2006 Biosens. Bioelectron. 21 1791
Kang I, Shin W-S, Manivannan S, Seo Y and Kim K 2016 J. Electrochem. Sci. Technol. 7 277
Gerber S J and Erasmus E 2018 Transit. Met. Chem. 43 409
Talukder P, Shit S, Noth H, Westerhausen M, Kneifel A N and Mitra S 2012 Transit. Met. Chem. 37 71
Sandu I, Vlaicu I, Cristian A and Mihailciuc C 2008 An. Univ. Bucuresti 2 25
Wang C, Zhang L, Guo Z, Xu J, Wang H, Zhai K et al 2010 Microchim. Acta 169 1
Shankaran D R and Narayanan S S 2001 Russ. J. Electrochem. 37 1149
Li J, Xie H and Chen L 2011 Sens. Actuators B 153 239
Qu F, Yang M, Lu Y, Shen G and Yu R 2006 Anal. Bioanal. Chem. 386 228
Wu X, Wang B, Li S, Liu J and Yu M 2015 RSC Adv. 5 33438
Huang Z, Xi L, Subhani Q, Yan W, Guo W and Zhu Y 2013 Carbon 62 127
Cumba L R, de Bicalho U O and do Carmo D R 2012 Int. J. Electrochem. Sci. 7 2123
Berrettoni M, Giorgetti M, Zamponi S, Conti P, Ranganathan D, Zanotto A et al 2010 J. Phys. Chem. C 114 6401
Shi Y, Zhou B, Wu P, Wang K and Cai C 2007 J. Electroanal. Chem. 611 1
Kaniyoor A and Ramaprabhu S 2012 J. Mater. Chem. 22 8377
Gupta R, Rastogi P K, Ganesan V, Yadav D K and Sonkar P K 2017 Sens. Actuators B 239 970
Gupta R, Rastogi P K, Srivastava U, Ganesan V, Sonkar P K and Yadav D K 2016 RSC Adv. 6 65779
Guo C X, Lei Y and Li C M 2011 Electroanalysis 23 885
Moon I K, Lee J, Ruoff R S and Lee H 2010 Nat. Commun. 1 73
Deng K, Li C, Qiu X, Zhou J and Hou Z 2015 Electrochim. Acta 174 1096
Maleki N, Safavi A, Farjami E and Tajabadi F 2008 Anal. Chim. Acta 611 151
Yang H, Lu B, Guo L and Qi B 2011 J. Electroanal. Chem. 650 171
Zare H R, Nasirizadeh N, Chatraei F and Makarem S 2009 Electrochim. Acta 54 2828
Ramaraj S, Sakthivel R, Chen S-M, Palanisamy S, Velusamy V, Chen T-W et al 2017 Int. J. Electrochem. Sci. 12 5567
Hosseini H, Ahmar H, Dehghani A, Bagheri A, Fakhari A R and Amini M M 2013 Electrochim. Acta 88 301
Liu Y, Li Y and He X 2014 Anal. Chim. Acta 819 26
Karimi-Maleh H, Moazampour M, Ensafi A A, Mallakpour S and Hatami M 2014 Environ. Sci. Pollut. Res. 21 5879
Zhu J, Chauhan D S, Shan D, Wu X-Y, Zhang G-Y and Zhang X-J 2014 Microchim. Acta 181 813
Acknowledgements
DST-ASEAN (IMRC/AISTDF/R&D/P-16/2018) programme is acknowledged for financial support. MY is thankful to CSIR, New Delhi for the senior research fellowship (09/013(0855)/2018-EMR-I). We thank Ms Mamta Patel for her assistance during the initial stage of this study in the electro-deposition and characterization of GO-CoHCF films and preliminary assessment of hydrazine determination. We are thankful to Prof O N Srivastava, Department of Physics, Banaras Hindu University for SEM facility.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Yadav, M., Ganesan, V., Gupta, R. et al. Amperometric assay of hydrazine utilizing electro-deposited cobalt hexacyanoferrate nanocrystals on graphene oxide sheets. Bull Mater Sci 43, 245 (2020). https://doi.org/10.1007/s12034-020-02219-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12034-020-02219-y