Abstract
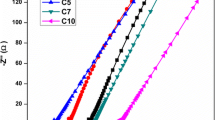
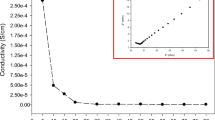
Composite polymer electrolytes based on poly(ethylene glycol) (PEG), magnesium acetate [Mg(CH3COO)2], and x wt% of cerium oxide (CeO2) ceramic fillers (where x = 0, 5, 10, 15 and 20, respectively) have been prepared using solution casting technique. X-ray diffraction patterns of PEG–Mg(CH3COO)2 with CeO 2 ceramic filler indicated the decrease in the degree of crystallinity with increasing concentration of the filler. DSC measurements of PEG–Mg(CH3COO)2–CeO2 composite polymer electrolyte system showed that the melting temperature is shifted towards the lower temperature with increase of the filler concentration. The conductivity results indicate that the incorporation of ceramic filler up to a certain concentration (i.e. 15 wt%) increases the ionic conductivity and upon further addition the conductivity decreases. The transference number data indicated the dominance of ion-type charge transport in these specimens. Using this (PEG–Mg(CH3COO)2–CeO2) (85-15-15) electrolyte, solid-state electrochemical cell was fabricated and their discharge profiles were studied under a constant load of 100 kΩ.








Similar content being viewed by others
References
Ash B J, Schadler L S and Siegel R W 2002 Mater. Lett. 55 83
Capiglia C, Mustarelli P, Quartarone E, Tomassi C and Magistris A 1999 Solid State Ionics 118 73
Chandra A, Srivastava P C and Chandra S 1995 J. Mater. Sci. 30 3633
Cho J and Liu M 1997 Electrochim. Acta 42 1481
Choi B K, Kim Y W and Shin K H 1997 J. Power Sources 68 357
Croce F, Appetecchi G B, Persi L and Scrosati B 1998 Nature 394 456
Croce F and Scrosati B 1993 J. Power Sources 43 9
Gregory T D, Hoffman R J and Winterton R C 1990 J. Electrochem. Soc. 137 775
Groce F, Persi L, Scrosati B, Serraino-Fiory F, Plishta E and Hendrickson M A 2001 Electrochim. Acta 46 2457
Jaipal Reddy M, Sreepathi Rao S, Laxmi Narasaiah E and Subba Rao U V 1995 Solid State Ionics 80 93
Jonscher A K 1977 Nature 267 673
Kumar B and Scanlon L G 1994 J. Power Sources 52 261
Macdonald J R 1987 Impedance spectroscopy, emphasizing solid materials and systems. WileyNew York
Matsuo Y and Kuwano J 1995 Solid State Ionics 79 295
Morita M, Fujisaki T, Yoshimoto N and Ishikawa M 2001 Electrochim. Acta 46 1565
Munichandraiah N, Scanlon L G, Marsh R A, Kumar B and Sircar A K 1995 J. Appl. Electrochem. 25 857
Peled E, Golodnitsky D, Ardel G and Eshkenazy V 1995 Electrochim. Acta 40 2197
Plocharski J, Wieczorek W, Przyluski J and Such K 1989 Appl. Phys. A49 55
Polu A R and Kumar R 2011 E-J. Chem. 8 347
Polu A R and Kumar R 2012 E-J. Chem. 9 869
Polu A R and Kumar R 2013 Internnat. J. Polym. Mater. 62 76
Polu A R, Kumar R and Dehariya H 2012 AIP Conf. Proc. 1447 969
Polu A R, Kumar R, Causin V and Neppalli R 2011 J. Korean Phys. Soc. 59 114
Przyluski J, Sickierski M and Wieczorek W 1995 Electrochim. Acta 40 2101
Przyluski J and Wieczorek W 1989 Solid State Ionics 36 165
Rajendran S and Uma T 2000a J. Power Sources 88 282
Rajendran S and Uma T 2000b Mater. Lett. 44 208
Ramalingaiah S, Srinivas Reddy D, Jaipal Reddy M, Laxmi Narasaiah E and Subba Rao U V 1996 Mater. Lett. 29 285
Robinson J L 1976 The primary battery Vol. II, (eds) N C Cahoon and G W Heise (New York: Wiley) p. 149
Sekhon S S and Sandhar G S 1998 Eur. Poly. J. 34 435
Sreepathi Rao S, Jaipal Reddy M, Laxmi Narasaiah E and Subba Rao U V 1995 Mater. Sci. Eng. B33 173
Subba Reddy Ch V, Sharma A K and Narasimha Rao V V R 2004 Ionics 10 142
Vijayakumar G, Karthick S N, Sathiya Priya A R, Ramalingam S and Subramania A J 2008 Solid State Electrochem. 12 1135
Wagner J B and Wagner C J 1957 Chem. Phys. 26 1597
Weston J E and Steele B C H 1982 Solid State Ionics 7 75
Wieczorek W 1992 Mater. Sci. Eng. B15 108
Wieczorek W, Florjanczyk Z and Stevens J R 1995 Electrochim. Acta 40 2251
Wieczorek W, Such K, Wycislik H and Plocharski J 1989 Solid State Ionics 36 255
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
POLU, A.R., KUMAR, R. Preparation and characterization of PEG–Mg(CH3COO)2–CeO2 composite polymer electrolytes for battery application. Bull Mater Sci 37, 309–314 (2014). https://doi.org/10.1007/s12034-014-0654-5
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12034-014-0654-5