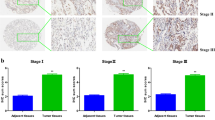
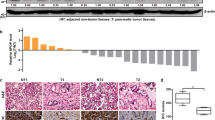
Abstract
PEST-containing nuclear protein (PCNP), a short-lived small nuclear protein with 178 amino acids, is a nuclear protein containing two PEST sequences. PCNP is highly expressed in several malignant tumors such as cervical cancer, rectal cancer, and lung cancer. It is also associated with cell cycle regulation and the phosphoinositide 3-kinase/protein kinase B/mammalian target of rapamycin (PI3K/AKT/mTOR) and Wnt signaling pathways during tumor growth. The present article discuss how PCNP regulates the PI3K/AKT/mTOR and Wnt signaling pathways and related proteins, and the ubiquitination of PCNP regulates tumor cell cycle as well as the progress of the application of PCNP in the pathophysiology and treatment of colon cancer, human ovarian cancer, thyroid cancer, lung adenocarcinoma and oral squamous cell carcinoma. The main relevant articles were retrieved from PubMed, with keywords such as PEST-containing nuclear protein (PCNP), cancer (tumor), and signaling pathways as inclusion/exclusion criteria. Relevant references has been included and cited in the manuscript.


Similar content being viewed by others
Data Availability
Data availability are not applicable to this review article.
References
Sarfraz, M., Afzal, A., Khattak, S., Saddozai, U. A. K., Li, H. M., Zhang, Q. Q., et al. (2021). Multifaceted behavior of PEST sequence enriched nuclear proteins in cancer biology and role in gene therapy. Journal of Cellular Physiology, 236(1), 1658–1676. https://doi.org/10.1002/jcp.30011
Mohamed, O. A. A., Tesen, H. S., Hany, M., Sherif, A., Abdelwahab, M. M., & Elnaggar, M. H. (2023). The role of hypoxia on prostate cancer progression and metastasis. Molecular Biology Reports, 50(1), 3873–3884. https://doi.org/10.1007/s11033-023-08251-5
Itkonen, H. M., Minner, S., Guldvik, I. J., Sandmann, M. J., Tsourlakis, M. C., Berge, V., et al. (2013). O-GlcNAc transferase integrates metabolic pathways to regulate the stability of c-MYC in human prostate cancer cells. Cancer Research, 73(1), 5277–5287. https://doi.org/10.1158/0008-5472.Can-13-0549
Rechsteiner, M. (1990). PEST sequences are signals for rapid intracellular proteolysis. Semin Cell Biol., 1(1), 433–40.
Rechsteiner, M., & Rogers, S. W. (1996). PEST sequences and regulation by proteolysis. Trends in Biochemical Sciences, 21(1), 267–271.
Mori, T., Li, Y., Hata, H., Ono, K., & Kochi, H. (2002). NIRF, a novel RING finger protein, is involved in cell-cycle regulation. Biochemical and Biophysical Research Communications, 296(1), 530–536. https://doi.org/10.1016/s0006-291x(02)00890-2
Khan, N. H., Chen, H. J., Fan, Y., Surfaraz, M., Ahammad, M. F., Qin, Y. Z., et al. (2022). Biology of PEST-containing nuclear protein: A potential molecular target for cancer research. Front Oncol., 12(1), 784597. https://doi.org/10.3389/fonc.2022.784597
Fang, T., Jiao, Z., You, Y., Cao, J., Wang, C., Liu, J., et al. (2023). Lenvatinib inhibited HCC cell migration and invasion through regulating the transcription and ubiquitination of UHRF1 and DNMT1. Biochemical Pharmacology, 210(1), 115489. https://doi.org/10.1016/j.bcp.2023.115489
Qian, G., Hu, B., Zhou, D., Xuan, Y., Bai, L., & Duan, C. (2015). NIRF, a novel ubiquitin ligase, inhibits hepatitis B virus replication through effect on HBV core protein and H3 histones. DNA Cell Biology, 34(1), 327–332. https://doi.org/10.1089/dna.2014.2714
Li, Y., Mori, T., Hata, H., Homma, Y., & Kochi, H. (2004). NIRF induces G1 arrest and associates with Cdk2. Biochemical and Biophysical Research Communications, 319(1), 464–468. https://doi.org/10.1016/j.bbrc.2004.04.190
Fu, H., Xing, F., Lv, Y., Zeng, B., You, P., & Liu, J. (2018). ICBP90 mediates Notch signaling to facilitate human hepatocellular carcinoma growth. Tissue Cell., 54(1), 65–71. https://doi.org/10.1016/j.tice.2018.08.004
Mousli, M., Hopfner, R., Abbady, A. Q., Monté, D., Jeanblanc, M., Oudet, P., et al. (2003). ICBP90 belongs to a new family of proteins with an expression that is deregulated in cancer cells. British Journal of Cancer, 89(1), 120–127. https://doi.org/10.1038/sj.bjc.6601068
Ni, X., Li, Z., Li, X., Zhang, X., Bai, G., Liu, Y., et al. (2022). Socioeconomic inequalities in cancer incidence and access to health services among children and adolescents in China: A cross-sectional study. Lancet., 400(1), 1020–1032. https://doi.org/10.1016/s0140-6736(22)01541-0
Wu, D. D., Gao, Y. R., Li, T., Wang, D. Y., Lu, D., Liu, S. Y., et al. (2018). PEST-containing nuclear protein mediates the proliferation, migration, and invasion of human neuroblastoma cells through MAPK and PI3K/AKT/mTOR signaling pathways. BMC Cancer., 18(1), 499. https://doi.org/10.1186/s12885-018-4391-9
Dong, P., Fu, H., Chen, L., Zhang, S., Zhang, X., Li, H., et al. (2020). PCNP promotes ovarian cancer progression by accelerating β-catenin nuclear accumulation and triggering EMT transition. Journal of Cellular and Molecular Medicine, 24(1), 8221–8235. https://doi.org/10.1111/jcmm.15491
Wang, D. Y., Hong, Y., Chen, Y. G., Dong, P. Z., Liu, S. Y., Gao, Y. R., et al. (2019). PEST-containing nuclear protein regulates cell proliferation, migration, and invasion in lung adenocarcinoma. Oncogenesis., 8(1), 22. https://doi.org/10.1038/s41389-019-0132-4
Mori, T., Li, Y., Hata, H., & Kochi, H. (2004). NIRF is a ubiquitin ligase that is capable of ubiquitinating PCNP, a PEST-containing nuclear protein. FEBS Letters, 557(1), 209–214. https://doi.org/10.1016/s0014-5793(03)01495-9
Mansour, M. A. (2018). Ubiquitination: Friend and foe in cancer. The International Journal of Biochemistry & Cell Biology, 101(1), 80–93. https://doi.org/10.1016/j.biocel.2018.06.001
Popovic, D., Vucic, D., & Dikic, I. (2014). Ubiquitination in disease pathogenesis and treatment. Nature Medicine, 20(1), 1242–1253. https://doi.org/10.1038/nm.3739
Chen, Z., & Lu, W. (2015). Roles of ubiquitination and SUMOylation on prostate cancer: Mechanisms and clinical implications. International Journal of Molecular Sciences, 16(1), 4560–4580. https://doi.org/10.3390/ijms16034560
Bassermann, F., Eichner, R., & Pagano, M. (2014). The ubiquitin proteasome system—implications for cell cycle control and the targeted treatment of cancer. Biochim Biophys Acta., 1843(1), 150–162. https://doi.org/10.1016/j.bbamcr.2013.02.028
Obaya, A. J., & Sedivy, J. M. (2002). Regulation of cyclin-Cdk activity in mammalian cells. Cellular and Molecular Life Sciences (CMLS), 59(1), 126–142. https://doi.org/10.1007/s00018-002-8410-1
Kölling, R., & Losko, S. (1997). The linker region of the ABC-transporter Ste6 mediates ubiquitination and fast turnover of the protein. The EMBO Journal., 16(1), 2251–2261. https://doi.org/10.1093/emboj/16.9.2251
Hicke, L., & Riezman, H. (1996). Ubiquitination of a yeast plasma membrane receptor signals its ligand-stimulated endocytosis. Cell., 84(1), 277–287. https://doi.org/10.1016/s0092-8674(00)80982-4
Wang, F., Zhang, P., Ma, Y., Yang, J., Moyer, M. P., Shi, C., et al. (2012). NIRF is frequently upregulated in colorectal cancer and its oncogenicity can be suppressed by let-7a microRNA. Cancer Lett., 314(1), 223–231. https://doi.org/10.1016/j.canlet.2011.09.033
Tewari, D., Patni, P., Bishayee, A., Sah, A. N., & Bishayee, A. (2022). Natural products targeting the PI3K-Akt-mTOR signaling pathway in cancer: A novel therapeutic strategy. Seminars in Cancer Biology, 80(1), 1–17. https://doi.org/10.1016/j.semcancer.2019.12.008
Mohite, R., & Doshi, G. (2023). Elucidation of the role of the epigenetic regulatory mechanisms of PI3K/AKT/mtor signaling pathway in human malignancies. Current Cancer Drug Targets. https://doi.org/10.2174/1568009623666230801094826
Ahmad, I., Hoque, M., Alam, S. S. M., Zughaibi, T. A., & Tabrez, S. (2023). Curcumin and plumbagin synergistically target the PI3K/Akt/mTOR Pathway: A prospective role in cancer treatment. Int J Mol Sci. https://doi.org/10.3390/ijms24076651
Jiang, B. H., & Liu, L. Z. (2008). PI3K/PTEN signaling in tumorigenesis and angiogenesis. Biochimica et Biophysica Acta (BBA) - Proteins and Proteomics, 1784(1), 150–158. https://doi.org/10.1016/j.bbapap.2007.09.008
Xu, F., Na, L., Li, Y., & Chen, L. (2020). Roles of the PI3K/AKT/mTOR signalling pathways in neurodegenerative diseases and tumours. Cell & Bioscience, 10(1), 54. https://doi.org/10.1186/s13578-020-00416-0
Ersahin, T., Tuncbag, N., & Cetin-Atalay, R. (2015). The PI3K/AKT/mTOR interactive pathway. Molecular BioSystems, 11(1), 1946–1954. https://doi.org/10.1039/c5mb00101c
Shi, N., Yu, H., & Chen, T. (2019). Inhibition of esophageal cancer growth through the suppression of PI3K/AKT/mTOR signaling pathway. OncoTargets and Therapy, 12(1), 7637–7647. https://doi.org/10.2147/ott.S205457
Trotman, L. C., Wang, X., Alimonti, A., Chen, Z., Teruya-Feldstein, J., Yang, H., et al. (2007). Ubiquitination regulates PTEN nuclear import and tumor suppression. Cell., 128(1), 141–156. https://doi.org/10.1016/j.cell.2006.11.040
Tang, Y., Luo, J., Zhou, Y., Zang, H., Yang, Y., Liu, S., et al. (2022). Overexpressed p-S6 associates with lymph node metastasis and predicts poor prognosis in non-small cell lung cancer. BMC Cancer., 22(1), 564. https://doi.org/10.1186/s12885-022-09664-4
Jiang, B. H., & Liu, L. Z. (2009). PI3K/PTEN signaling in angiogenesis and tumorigenesis. Advanced Cancer Research, 102(1), 19–65. https://doi.org/10.1016/s0065-230x(09)02002-8
Yang, J., Pi, C., & Wang, G. (2018). Inhibition of PI3K/Akt/mTOR pathway by apigenin induces apoptosis and autophagy in hepatocellular carcinoma cells. Biomedicine & Pharmacotherapy, 103(1), 699–707. https://doi.org/10.1016/j.biopha.2018.04.072
Aggarwal, S., John, S., Sapra, L., Sharma, S. C., & Das, S. N. (2019). Targeted disruption of PI3K/Akt/mTOR signaling pathway, via PI3K inhibitors, promotes growth inhibitory effects in oral cancer cells. Cancer Chemother Pharmacol., 83(1), 451–461. https://doi.org/10.1007/s00280-018-3746-x
Liu, S., Gao, W., Lu, Y., Zhou, Q., Su, R., Hasegawa, T., et al. (2022). As a novel tumor suppressor, LHPP promotes apoptosis by inhibiting the PI3K/AKT signaling pathway in oral squamous cell carcinoma. International Journal of Biological Sciences, 18(1), 491–506. https://doi.org/10.7150/ijbs.66841
Wang, X., & Chen, Q. (2021). FERMT1 knockdown inhibits oral squamous cell carcinoma cell epithelial-mesenchymal transition by inactivating the PI3K/AKT signaling pathway. BMC Oral Health., 21(1), 598. https://doi.org/10.1186/s12903-021-01955-9
Liu, W., Wang, J., Zhang, C., Bao, Z., & Wu, L. (2022). Curcumin nanoemulsions inhibit oral squamous cell carcinoma cell proliferation by PI3K/Akt/mTOR suppression and miR-199a upregulation: A preliminary study. Oral disease. https://doi.org/10.1111/odi.14271
Yoon, J. H., Shin, J. W., Pham, T. H., Choi, Y. J., Ryu, H. W., Oh, S. R., et al. (2020). Methyl lucidone induces apoptosis and G(2)/M phase arrest via the PI3K/Akt/NF-κB pathway in ovarian cancer cells. Pharmaceutical Biology, 58(1), 51–59. https://doi.org/10.1080/13880209.2019.1701044
Luo, G., Zhou, J., Li, G., Hu, N., Xia, X., & Zhou, H. (2021). Retracted: Ferruginol diterpenoid selectively inhibits human thyroid cancer growth by inducing mitochondrial dependent apoptosis, endogenous reactive oxygen species (ROS) production, mitochondrial membrane potential loss and suppression of mitogen-activated protein kinase (MAPK) and PI3K/AKT signaling pathways. Medical Science Monitor, 27(1), e932341. https://doi.org/10.12659/msm.932341
Wang, Y., Xu, J., Alarifi, S., & Wang, H. (2021). Kirenol inhibited the cell survival and induced apoptosis in human thyroid cancer cells by altering PI3K/AKT and MAP kinase signaling pathways. Environmental Toxicology, 36(1), 811–820. https://doi.org/10.1002/tox.23083
Ghahhari, N. M., & Babashah, S. (2015). Interplay between microRNAs and WNT/β-catenin signalling pathway regulates epithelial-mesenchymal transition in cancer. European Journal of Cancer, 51(1), 1638–1649. https://doi.org/10.1016/j.ejca.2015.04.021
Clevers, H. (2006). Wnt/beta-catenin signaling in development and disease. Cell., 127(1), 469–480. https://doi.org/10.1016/j.cell.2006.10.018
Cheng, X., Xu, X., Chen, D., Zhao, F., & Wang, W. (2019). Therapeutic potential of targeting the Wnt/β-catenin signaling pathway in colorectal cancer. Biomedicine & Pharmacotherapy, 110(1), 473–481. https://doi.org/10.1016/j.biopha.2018.11.082
Degirmenci, B., Dincer, C., Demirel, H. C., Berkova, L., Moor, A. E., Kahraman, A., et al. (2021). Epithelial Wnt secretion drives the progression of inflammation-induced colon carcinoma in murine model. iScience., 24(1), 103369. https://doi.org/10.1016/j.isci.2021.103369
Hall, C. L., Kang, S., MacDougald, O. A., & Keller, E. T. (2006). Role of Wnts in prostate cancer bone metastases. Journal of Cellular Biochemistry, 97(1), 661–672. https://doi.org/10.1002/jcb.20735
Hall, C. L., & Keller, E. T. (2006). The role of Wnts in bone metastases. Cancer and Metastasis Reviews, 25(1), 551–558. https://doi.org/10.1007/s10555-006-9022-2
Barbolina, M. V., Burkhalter, R. J., & Stack, M. S. (2011). Diverse mechanisms for activation of Wnt signalling in the ovarian tumour microenvironment. Biochemical Journal., 437(1), 1–12. https://doi.org/10.1042/bj20110112
Kramer, E. D., Tzetzo, S. L., Colligan, S. H., Hensen, M. L., Brackett, C. M., Clausen, B. E., et al. (2023). β-Catenin signaling in alveolar macrophages enhances lung metastasis through a TNF-dependent mechanism. JCI Insight. https://doi.org/10.1172/jci.insight.160978
Zhou, Y., Xu, J., Luo, H., Meng, X., Chen, M., & Zhu, D. (2022). Wnt signaling pathway in cancer immunotherapy. Cancer Letters, 525(1), 84–96. https://doi.org/10.1016/j.canlet.2021.10.034
Liu, J., Xiao, Q., Xiao, J., Niu, C., Li, Y., Zhang, X., et al. (2022). Wnt/β-catenin signalling: Function, biological mechanisms, and therapeutic opportunities. Signal Transduction and Targeted Therapy, 7(1), 3. https://doi.org/10.1038/s41392-021-00762-6
Dooling, L. J., Andrechak, J. C., Hayes, B. H., Kadu, S., Zhang, W., Pan, R., et al. (2023). Cooperative phagocytosis of solid tumours by macrophages triggers durable anti-tumour responses. Nature Biomedical Engineering. https://doi.org/10.1038/s41551-023-01031-3
Chen, T., You, Y., Jiang, H., & Wang, Z. Z. (2017). Epithelial-mesenchymal transition (EMT): A biological process in the development, stem cell differentiation, and tumorigenesis. Journal of Cellular Physiology, 232(1), 3261–3272. https://doi.org/10.1002/jcp.25797
Zhang, Y., & Weinberg, R. A. (2018). Epithelial-to-mesenchymal transition in cancer: Complexity and opportunities. Frontiers of Medicine, 12(1), 361–373. https://doi.org/10.1007/s11684-018-0656-6
Yu, F., Yu, C., Li, F., Zuo, Y., Wang, Y., Yao, L., et al. (2021). Wnt/β-catenin signaling in cancers and targeted therapies. Signal Transduction and Targeted Therapy, 6(1), 307. https://doi.org/10.1038/s41392-021-00701-5
Arend, R. C., Londoño-Joshi, A. I., Straughn, J. M., Jr., & Buchsbaum, D. J. (2013). The Wnt/β-catenin pathway in ovarian cancer: A review. Gynecologic Oncology, 131(1), 772–779. https://doi.org/10.1016/j.ygyno.2013.09.034
Chen, Y. G., Liu, H. X., Hong, Y., Dong, P. Z., Liu, S. Y., Gao, Y. R., et al. (2022). PCNP is a novel regulator of proliferation, migration, and invasion in human thyroid cancer. International Journal of Biological Sciences, 18(1), 3605–3620. https://doi.org/10.7150/ijbs.70394
Mori, T., Ikeda, D. D., Fukushima, T., Takenoshita, S., & Kochi, H. (2011). NIRF constitutes a nodal point in the cell cycle network and is a candidate tumor suppressor. Cell Cycle., 10(1), 3284–3299. https://doi.org/10.4161/cc.10.19.17176
Mori, T., Ikeda, D. D., Yamaguchi, Y., & Unoki, M. (2012). NIRF/UHRF2 occupies a central position in the cell cycle network and allows coupling with the epigenetic landscape. FEBS Letters, 586(1), 1570–1583. https://doi.org/10.1016/j.febslet.2012.04.038
van Noort, V., Snel, B., & Huynen, M. A. (2003). Predicting gene function by conserved co-expression. Trends in Genetics, 19(1), 238–242. https://doi.org/10.1016/s0168-9525(03)00056-8
Saris, C. G., Horvath, S., van Vught, P. W., van Es, M. A., Blauw, H. M., Fuller, T. F., et al. (2009). Weighted gene co-expression network analysis of the peripheral blood from amyotrophic lateral sclerosis patients. BMC Genomics., 10(1), 405. https://doi.org/10.1186/1471-2164-10-405
Li, Z., Zheng, W., Wang, Z., Zeng, Z., Zhan, H., Li, C., et al. (2013). A transgenic zebrafish liver tumor model with inducible Myc expression reveals conserved Myc signatures with mammalian liver tumors. Disease Models & Mechanisms., 6(1), 414–423. https://doi.org/10.1242/dmm.010462
de Jong, S., Newhouse, S. J., Patel, H., Lee, S., Dempster, D., Curtis, C., et al. (2016). Immune signatures and disorder-specific patterns in a cross-disorder gene expression analysis. British Journal of Psychiatry., 209(1), 202–208. https://doi.org/10.1192/bjp.bp.115.175471
Kim, S., Westphal, V., Srikrishna, G., Mehta, D. P., Peterson, S., Filiano, J., et al. (2000). Dolichol phosphate mannose synthase (DPM1) mutations define congenital disorder of glycosylation Ie (CDG-Ie). Journal of Clinical Investigation., 105(1), 191–198. https://doi.org/10.1172/jci7302
Bard, J. A. M., Goodall, E. A., Greene, E. R., Jonsson, E., Dong, K. C., & Martin, A. (2018). Structure and function of the 26S proteasome. Annual Review of Biochemistry, 87(1), 697–724. https://doi.org/10.1146/annurev-biochem-062917-011931
Ng, C. L., Oresic, K., & Tortorella, D. (2010). TRAM1 is involved in disposal of ER membrane degradation substrates. Experimental Cell Research, 316(1), 2113–2122. https://doi.org/10.1016/j.yexcr.2010.04.010
Wang, H., Lee, H. W., Deng, Y., Lu, Z., Hsu, P. C., Liu, Y., et al. (2015). Bifunctional non-noble metal oxide nanoparticle electrocatalysts through lithium-induced conversion for overall water splitting. Nature Communications, 6(1), 7261. https://doi.org/10.1038/ncomms8261
Hu, J., Zhang, L., Mei, Z., Jiang, Y., Yi, Y., Liu, L., et al. (2018). Interaction of E3 ubiquitin ligase MARCH7 with long noncoding RNA MALAT1 and autophagy-related protein ATG7 promotes autophagy and invasion in ovarian cancer. Cellular Physiology and Biochemistry, 47(1), 654–666. https://doi.org/10.1159/000490020
Zhao, K., Yang, Y., Zhang, G., Wang, C., Wang, D., Wu, M., et al. (2018). Regulation of the Mdm2-p53 pathway by the ubiquitin E3 ligase MARCH7. EMBO Reports., 19(1), 305–319. https://doi.org/10.15252/embr.201744465
Demirel, D., Ozkaya, F. C., Ebrahim, W., Sokullu, E., & Sahin, I. D. (2023). Aspergillus carneus metabolite averufanin induced cell cycle arrest and apoptotic cell death on cancer cell lines via inducing DNA damage. Scientific Reports, 13(1), 6460. https://doi.org/10.1038/s41598-023-30775-w
Roslan, N. H., Makpol, S., & Mohd Yusof, Y. A. (2019). A review on dietary intervention in obesity associated colon cancer. Asian Pacific Journal of Cancer Prevention, 20(1), 1309–1319. https://doi.org/10.31557/apjcp.2019.20.5.1309
Liu, K., Lai, M., Wang, S., Zheng, K., Xie, S., & Wang, X. (2020). Construction of a CXC chemokine-based prediction model for the prognosis of colon cancer. BioMed Research International, 1, 6107865. https://doi.org/10.1155/2020/6107865
Bray, F., Ferlay, J., Soerjomataram, I., Siegel, R. L., Torre, L. A., & Jemal, A. (2018). Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer Journal for Clinicians., 68(1), 394–424. https://doi.org/10.3322/caac.21492
Liu, F., Wang, X. D., & Du, S. Y. (2020). Production of gold/silver doped carbon nanocomposites for effective photothermal therapy of colon cancer. Scientific Reports., 10(1), 7618. https://doi.org/10.1038/s41598-020-64225-8
Verhoeven, Y., Tilborghs, S., Jacobs, J., De Waele, J., Quatannens, D., Deben, C., et al. (2020). The potential and controversy of targeting STAT family members in cancer. Seminars in Cancer Biology, 60(1), 41–56. https://doi.org/10.1016/j.semcancer.2019.10.002
Tuli, H. S., Sak, K., Iqubal, A., Garg, V. K., Varol, M., Sharma, U., et al. (2022). STAT signaling as a target for intervention: From cancer inflammation and angiogenesis to non-coding RNAs modulation. Molecular Biology Reports, 49(1), 8987–8999. https://doi.org/10.1007/s11033-022-07399-w
Slattery, M. L., Lundgreen, A., Kadlubar, S. A., Bondurant, K. L., & Wolff, R. K. (2013). JAK/STAT/SOCS-signaling pathway and colon and rectal cancer. Mol Carcinog., 52(1), 155–166. https://doi.org/10.1002/mc.21841
Han, S. W., Ahn, J. Y., Lee, S., Noh, Y. S., Jung, H. C., Lee, M. H., et al. (2020). Gene expression network analysis of lymph node involvement in colon cancer identifies AHSA2, CDK10, and CWC22 as possible prognostic markers. Scientific Reports, 10(1), 7170. https://doi.org/10.1038/s41598-020-63806-x
Xu, T., Wu, K., Shi, J., Ji, L., Song, X., Tao, G., et al. (2022). LINC00858 promotes colon cancer progression through activation of STAT3/5 signaling by recruiting transcription factor RAD21 to upregulate PCNP. Cell Death Discovery, 8(1), 228. https://doi.org/10.1038/s41420-022-00832-w
Yue, B., Liu, C., Sun, H., Liu, M., Song, C., Cui, R., et al. (2018). A positive feed-forward loop between LncRNA-CYTOR and Wnt/β-catenin signaling promotes metastasis of colon cancer. Molecular Therapy, 26(1), 1287–1298. https://doi.org/10.1016/j.ymthe.2018.02.024
Zhao, H., Ming, T., Tang, S., Ren, S., Yang, H., Liu, M., et al. (2022). Wnt signaling in colorectal cancer: Pathogenic role and therapeutic target. Molecular Cancer., 21(1), 144. https://doi.org/10.1186/s12943-022-01616-7
Liu, F., Liang, Y., Sun, R., Yang, W., Liang, Z., Gu, J., et al. (2022). Astragalus mongholicus Bunge and Curcuma aromatica Salisb. inhibits liver metastasis of colon cancer by regulating EMT via the CXCL8/CXCR2 axis and PI3K/AKT/mTOR signaling pathway. Chinese Medicine, 17(1), 91. https://doi.org/10.1186/s13020-022-00641-4
Narayanankutty, A. (2019). PI3K/ Akt/ mTOR pathway as a therapeutic target for colorectal cancer: A review of preclinical and clinical evidence. Current Drug Targets, 20(1), 1217–1226. https://doi.org/10.2174/1389450120666190618123846
Wang, R., Li, S., Hou, Q., Zhang, B., Chu, H., Hou, Y., et al. (2023). Propofol inhibits colon cancer cell stemness and epithelial-mesenchymal transition by regulating SIRT1, Wnt/β-catenin and PI3K/AKT/mTOR signaling pathways. Discover Oncology, 14(1), 137. https://doi.org/10.1007/s12672-023-00734-y
Zhu, L., Tian, G., Yang, Q., De, G., Zhang, Z., Wang, Y., et al. (2016). Thyroid hormone receptor β1 suppresses proliferation and migration by inhibiting PI3K/Akt signaling in human colorectal cancer cells. Oncology Reports, 36(1), 1419–1426. https://doi.org/10.3892/or.2016.4931
Zeng, S., Chen, L., Sun, Q., Zhao, H., Yang, H., Ren, S., et al. (2021). Scutellarin ameliorates colitis-associated colorectal cancer by suppressing Wnt/β-catenin signaling cascade. European Journal of Pharmacology, 906(1), 174253. https://doi.org/10.1016/j.ejphar.2021.174253
Liu, Y., Luo, Y., Cai, M., Shen, P., Li, J., Chen, H., et al. (2021). Anti-angiogenic therapy in ovarian cancer: Current situation and prospects. Indian Journal of Medical Research, 154(1), 680–690. https://doi.org/10.4103/ijmr.IJMR_1160_19
Penny, S. M. (2020). Ovarian cancer: An overview. Radiologic Technology, 91(1), 561–575.
Wilczyński, J. R., Wilczyński, M., & Paradowska, E. (2022). Cancer stem cells in ovarian cancer-a source of tumor success and a challenging target for novel therapies. International Journal of Molecular Sciences. https://doi.org/10.3390/ijms23052496
Gupta, K. K., Gupta, V. K., & Naumann, R. W. (2019). Ovarian cancer: Screening and future directions. International Journal of Gynecologic Cancer, 29(1), 195–200. https://doi.org/10.1136/ijgc-2018-000016
Menon, U., Griffin, M., & Gentry-Maharaj, A. (2014). Ovarian cancer screening–current status, future directions. Gynecologic Oncology, 132(1), 490–495. https://doi.org/10.1016/j.ygyno.2013.11.030
Kwon, M., Kim, J. H., Rybak, Y., Luna, A., Choi, C. H., Chung, J. Y., et al. (2016). Reduced expression of FILIP1L, a novel WNT pathway inhibitor, is associated with poor survival, progression and chemoresistance in ovarian cancer. Oncotarget, 7(1), 77052–77070. https://doi.org/10.18632/oncotarget.12784
Lu, R., Tang, P., Zhang, D., Lin, S., Li, H., Feng, X., et al. (2023). SOX9/NFIA promotes human ovarian cancer metastasis through the Wnt/β-catenin signaling pathway. Pathology - Research and Practice, 248(1), 154602. https://doi.org/10.1016/j.prp.2023.154602
Ruiz-Pozo, V. A., Cadena-Ullauri, S., Guevara-Ramírez, P., Paz-Cruz, E., Tamayo-Trujillo, R., & Zambrano, A. K. (2023). Differential microRNA expression for diagnosis and prognosis of papillary thyroid cancer. Frontiers in Medicine (Lausanne)., 10(1), 1139362. https://doi.org/10.3389/fmed.2023.1139362
Ahmed, A. A., & Essa, M. E. A. (2019). Potential of epigenetic events in human thyroid cancer. Cancer Genetics, 239(1), 13–21. https://doi.org/10.1016/j.cancergen.2019.08.006
Sung, H., Ferlay, J., Siegel, R. L., Laversanne, M., Soerjomataram, I., Jemal, A., et al. (2021). Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: Cancer Journal for Clinicians, 71(1), 209–249. https://doi.org/10.3322/caac.21660
Morris, L. G., Sikora, A. G., Tosteson, T. D., & Davies, L. (2013). The increasing incidence of thyroid cancer: The influence of access to care. Thyroid., 23(1), 885–891. https://doi.org/10.1089/thy.2013.0045
Yu, J., Deng, Y., Liu, T., Zhou, J., Jia, X., Xiao, T., et al. (2020). Lymph node metastasis prediction of papillary thyroid carcinoma based on transfer learning radiomics. Nature Communications, 11(1), 4807. https://doi.org/10.1038/s41467-020-18497-3
Hu, Q., Zhang, R., Zheng, J., Song, M., Gu, C., & Li, W. (2023). Hydrogen sulfide attenuates uranium-induced kidney cells pyroptosis via upregulation of PI3K/AKT/mTOR signaling. Journal of Biochemical and Molecular Toxicology, 37(1), e23220. https://doi.org/10.1002/jbt.23220
Wu, D., Wang, H., Teng, T., Duan, S., Ji, A., & Li, Y. (2018). Hydrogen sulfide and autophagy: A double edged sword. Pharmacological Research, 131(1), 120–127. https://doi.org/10.1016/j.phrs.2018.03.002
Wu, D., Li, J., Zhang, Q., Tian, W., Zhong, P., Liu, Z., et al. (2019). Exogenous hydrogen sulfide regulates the growth of human thyroid carcinoma cells. Oxidative Medicine and Cellular Longevity, 1, 6927298. https://doi.org/10.1155/2019/6927298
Chen, T., & Wong, Y. S. (2008). Selenocystine induces S-phase arrest and apoptosis in human breast adenocarcinoma MCF-7 cells by modulating ERK and Akt phosphorylation. Journal of Agricultural and Food Chemistry, 56(1), 10574–10581. https://doi.org/10.1021/jf802125t
Pang, R., Xu, Y., Hu, X., Liu, B., & Yu, J. (2020). Vitamin D receptor knockdown attenuates the antiproliferative, pro-apoptotic and anti-invasive effect of vitamin D by activating the Wnt/β-catenin signaling pathway in papillary thyroid cancer. Molecular Medicine Reports., 22(1), 4135–4142. https://doi.org/10.3892/mmr.2020.11522
Xin, S., & Ye, X. (2020). Knockdown of long non-coding RNA CCAT2 suppresses the progression of thyroid cancer by inhibiting the Wnt/β-catenin pathway. International Journal of Molecular Medicine, 46(1), 2047–2056. https://doi.org/10.3892/ijmm.2020.4761
Keith, R. L., & Miller, Y. E. (2013). Lung cancer chemoprevention: Current status and future prospects. Nature Reviews Clinical Oncology, 10(1), 334–343. https://doi.org/10.1038/nrclinonc.2013.64
Politi, K., & Herbst, R. S. (2015). Lung cancer in the era of precision medicine. Clinical Cancer Research, 21(1), 2213–2220. https://doi.org/10.1158/1078-0432.Ccr-14-2748
Lennon, F. E., Cianci, G. C., Cipriani, N. A., Hensing, T. A., Zhang, H. J., Chen, C. T., et al. (2015). Lung cancer-a fractal viewpoint. Nature Reviews Clinical Oncology, 12(1), 664–675. https://doi.org/10.1038/nrclinonc.2015.108
Kanodra, N. M., Silvestri, G. A., & Tanner, N. T. (2015). Screening and early detection efforts in lung cancer. Cancer., 121(1), 1347–1356. https://doi.org/10.1002/cncr.29222
Ferlay, J., Colombet, M., Soerjomataram, I., Mathers, C., Parkin, D. M., Piñeros, M., et al. (2019). Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. International Journal of Cancer, 144(1), 1941–1953. https://doi.org/10.1002/ijc.31937
Niculescu Talpoş, I. C., Rumel, R. C., Scurtu, A. D., Dinu, Ş, Miron, M. I., Preduţ, A. D., et al. (2021). Oral squamous cell carcinomas: A histopathological review of multiple cases from Western Romania. Romanian Journal of Morphology and Embryology, 62(1), 929–937. https://doi.org/10.47162/rjme.62.4.05
Rahman, R., Shaikh, M. H., Gopinath, D., Idris, A., & Johnson, N. W. (2023). Human papillomavirus and Epstein-Barr virus co-infection in oral and oropharyngeal squamous cell carcinomas: A systematic review and meta-analysis. Molecular Oral Microbiology, 38, 259–274. https://doi.org/10.1111/omi.12412
Chamoli, A., Gosavi, A. S., Shirwadkar, U. P., Wangdale, K. V., Behera, S. K., Kurrey, N. K., et al. (2021). Overview of oral cavity squamous cell carcinoma: Risk factors, mechanisms, and diagnostics. Oral Oncology, 121(1), 105451. https://doi.org/10.1016/j.oraloncology.2021.105451
Li, C. X., Liu, H., & Gong, Z. C. (2022). What is the potential interplay between microbiome and tumor microenvironment in oral squamous cell carcinomas? Asian Pacific Journal of Cancer Prevention, 23, 2199–2213. https://doi.org/10.31557/apjcp.2022.23.7.2199
Rorke, L. B. (1997). Pathologic diagnosis as the gold standard. Cancer., 79(1), 665–667. https://doi.org/10.1002/(sici)1097-0142(19970215)79:4%3c665::aid-cncr1%3e3.0.co;2-d
Zhang, L., Guo, D., Shen, J., Zheng, Y., Zhai, J., Li, R., et al. (2022). Tissue mechanics modulate PCNP expression in oral squamous cell carcinomas with different differentiation. Frontiers in Oncology, 12, 1072276. https://doi.org/10.3389/fonc.2022.1072276
Han, Z. P., Yang, Y., Chen, H. Y., Zhong, M. Y., & Zhuang, G. H. (2021). TIPE2 and PCNP expression abnormalities in peripheral blood mononuclear cells associated with disease activity in rheumatoid arthritis: A meta-analysis. European Review for Medical and Pharmacological Sciences, 25, 1242–1249. https://doi.org/10.26355/eurrev_202102_24828
Funding
This work was supported by the Program for Innovative Talents of Science and Technology in Henan Province (No. 23HASTIT043), the Natural Science Foundation of Henan Province for Excellent Young Scholars (No. 212300410026), the Medical Science and Technology Program of Henan Province (No. SBGJ202103096), and the Program for Young Key Teacher of Henan Province (No. 2020GGJS037).
Author information
Authors and Affiliations
Contributions
Conceptualization: SL and XJ; writing—original draft: SG, RD, QZ, and XW; writing—review and editing: SL and XJ.
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Guo, S., Ding, R., Zhao, Q. et al. Recent Insights into the Roles of PEST‐Containing Nuclear Protein. Mol Biotechnol (2024). https://doi.org/10.1007/s12033-024-01188-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12033-024-01188-5