Abstract
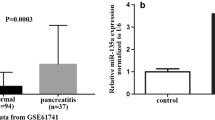
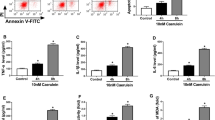
Acute pancreatitis (AP) is the most prevalent gastrointestinal inflammatory disease. Circular RNAs (circRNAs) are implicated in the development of AP. Here, we identified the precise action of circ_0029407 in AP development. Human pancreatic epithelial cells (HPECs) were stimulated with caerulein. Cell viability, proliferation, and apoptosis were gauged by Cell Counting Kit-8 (CCK-8), 5-ethynyl-2′-deoxyuridine (EdU), and flow cytometry assays, respectively. Circ_0029407, microRNA (miR)-579-3p, and toll-like receptor 4 (TLR4) were quantified by a qRT-PCR or western blot assay. Dual-luciferase reporter and RNA pull-down assays were performed to evaluate the direct relationship between miR-579-3p and circ_0029407 or TLR4. Our results indicated that circ_0029407 was markedly overexpressed in AP serum samples and caerulein-stimulated HPECs. Reduction of circ_0029407 attenuated caerulein-imposed HPEC damage by promoting cell proliferation and repressing cell apoptosis and inflammation. Mechanistically, circ_0029407 contained a miR-579-3p binding site, and miR-579-3p downregulation reversed the effect of circ_0029407 reduction on caerulein-imposed HPEC damage. TLR4 was identified as a direct and functional target of miR-579-3p, and TLR4 overexpression reversed the impact of miR-579-3p upregulation on attenuating caerulein-imposed HPEC damage. Moreover, circ_0029407 regulated the TLR4/nuclear factor NF-kappaB (NF-κB) signaling by acting as a competing endogenous RNA (ceRNA) for miR-579-3p. Our study suggests that circ_0029407 regulates caerulein-imposed cell injury in human pancreatic cells at least in part via the TLR4/NF-κB signaling pathway by functioning as a ceRNA for miR-579-3p.







Similar content being viewed by others
Data availability
Not applicable.
References
Hart, P. A., Bradley, D., Conwell, D. L., Dungan, K., Krishna, S. G., Wyne, K., Bellin, M. D., Yadav, D., Andersen, D. K., Serrano, J., & Papachristou, G. I. (2021). Diabetes following acute pancreatitis. The Lancet Gastroenterology and Hepatology, 6(8), 668–675. https://doi.org/10.1016/s2468-1253(21)00019-4
Hong, W., Pan, J., Goyal, H., & Zippi, M. (2023). Editorial: Acute pancreatitis infection: Epidemiology, prevention, clinical characteristics, treatment, and prediction. Frontiers in Cellular and Infection Microbiology, 13, 1175195. https://doi.org/10.3389/fcimb.2023.1175195
Iannuzzi, J. P., King, J. A., Leong, J. H., Quan, J., Windsor, J. W., Tanyingoh, D., Coward, S., Forbes, N., Heitman, S. J., Shaheen, A. A., Swain, M., Buie, M., Underwood, F. E., & Kaplan, G. G. (2022). Global incidence of acute pancreatitis is increasing over time: A systematic review and meta-analysis. Gastroenterology, 162(1), 122–134. https://doi.org/10.1053/j.gastro.2021.09.043
Petrov, M. S., & Yadav, D. (2019). Global epidemiology and holistic prevention of pancreatitis. Nature Reviews Gastroenterology & Hepatology, 16(3), 175–184. https://doi.org/10.1038/s41575-018-0087-5
Szatmary, P., Grammatikopoulos, T., Cai, W., Huang, W., Mukherjee, R., Halloran, C., Beyer, G., & Sutton, R. (2022). Acute pancreatitis: Diagnosis and treatment. Drugs, 82(12), 1251–1276. https://doi.org/10.1007/s40265-022-01766-4
Huang, H., Chen, W., Lu, J., Zhang, S., Xiang, X., Wang, X., & Tang, G. (2022). Circ_0000284 promoted acute pancreatitis progression through the regulation of miR-10a-5p/Wnt/β-catenin pathway. Chemistry & Biodiversity, 19(6), e202101006. https://doi.org/10.1002/cbdv.202101006
Chen, H., Tu, J., He, L., Gao, N., & Yang, W. (2023). Mmu_circ_0000037 inhibits the progression of acute pancreatitis by miR-92a-3p/Pias1 axis. Immunity, Inflammation and Disease, 11(4), e819. https://doi.org/10.1002/iid3.819
Bourgault, J., Abner, E., Manikpurage, H. D., Pujol-Gualdo, N., Laisk, T., Gobeil, É., Gagnon, E., Girard, A., Mitchell, P. L., Thériault, S., Esko, T., Mathieu, P., & Arsenault, B. J. (2023). Proteome-wide Mendelian randomization identifies causal links between blood proteins and acute pancreatitis. Gastroenterology, 164(6), 953-965.e953. https://doi.org/10.1053/j.gastro.2023.01.028
Kristensen, L. S., Jakobsen, T., Hager, H., & Kjems, J. (2022). The emerging roles of circRNAs in cancer and oncology. Nature Reviews Clinical Oncology, 19(3), 188–206. https://doi.org/10.1038/s41571-021-00585-y
Zhao, X., Zhong, Y., Wang, X., Shen, J., & An, W. (2022). Advances in circular RNA and its applications. International Journal of Medical Sciences, 19(6), 975–985. https://doi.org/10.7150/ijms.71840
Feng, M., Qin, B., Luo, F., Zhu, X., Liu, K., Li, K., Wu, D., Chen, G., & Tang, X. (2024). Qingjie Huagong decoction inhibits pancreatic acinar cell pyroptosis by regulating circHipk3/miR-193a-5p/NLRP3 pathway. Phytomedicine, 126, 155265. https://doi.org/10.1016/j.phymed.2023.155265
Liu, C., Zhu, X., Niu, X., Chen, L., & Ge, C. (2020). Elevated hsa_circRNA_101015, hsa_circRNA_101211, and hsa_circRNA_103470 in the human blood: Novel biomarkers to early diagnose acute pancreatitis. BioMed Research International, 2020, 2419163. https://doi.org/10.1155/2020/2419163
Ren, S., Pan, L., Yang, L., Niu, Z., Wang, L., Gao, Y., Liu, J., Liu, Z., & Pei, H. (2021). Interfering hsa_circ_0073748 alleviates caerulein-induced ductal cell injury in acute pancreatitis by inhibiting miR-132-3p/TRAF3/NF-κB pathway. Cell Cycle. https://doi.org/10.1080/15384101.2021.2014653
Yang, Q., Luo, Y., Ge, P., Lan, B., Liu, J., Wen, H., Cao, Y., Sun, Z., Zhang, G., Yuan, H., Zhang, L., & Chen, H. (2023). Emodin ameliorates severe acute pancreatitis-associated acute lung injury in rats by modulating exosome-specific miRNA expression profiles. International Journal of Nanomedicine, 18, 6743–6761. https://doi.org/10.2147/ijn.s428924
Li, X., Qin, H., Anwar, A., Zhang, X., Yu, F., Tan, Z., & Tang, Z. (2022). Molecular mechanism analysis of m6A modification-related lncRNA-miRNA-mRNA network in regulating autophagy in acute pancreatitis. Islets, 14(1), 184–199. https://doi.org/10.1080/19382014.2022.2132099
Desai, C. S., Khan, A., Bellio, M. A., Willis, M. L., Mahung, C., Ma, X., Baldwin, X., Williams, B. M., Baron, T. H., Coleman, L. G., Wallet, S. M., & Maile, R. (2021). Characterization of extracellular vesicle miRNA identified in peripheral blood of chronic pancreatitis patients. Molecular and Cellular Biochemistry, 476(12), 4331–4341. https://doi.org/10.1007/s11010-021-04248-5
Zhang, Y., Liang, X., Bao, X., Xiao, W., & Chen, G. (2022). Toll-like receptor 4 (TLR4) inhibitors: Current research and prospective. European Journal of Medicinal Chemistry, 235, 114291. https://doi.org/10.1016/j.ejmech.2022.114291
Wen, E., Xin, G., Su, W., Li, S., Zhang, Y., Dong, Y., Yang, X., Wan, C., Chen, Z., Yu, X., Zhang, K., Niu, H., & Huang, W. (2022). Activation of TLR4 induces severe acute pancreatitis-associated spleen injury via ROS-disrupted mitophagy pathway. Molecular Immunology, 142, 63–75. https://doi.org/10.1016/j.molimm.2021.12.012
Hu, Q., Tao, R., Hu, X., Wu, H., & Xu, J. (2023). Effects of piperlonguminine on lung injury in severe acute pancreatitis via the TLR4/NF-κB pathway. European Journal of Histochemistry. https://doi.org/10.4081/ejh.2023.3639
Fawzy, H. A., Mohammed, A. A., Fawzy, H. M., & Fikry, E. M. (2022). Reorienting of pramipexole as a promising therapy for acute pancreatitis in a rat model by suppressing TLR4\NF-κB p65\NLRP3 inflammasome signaling. Canadian Journal of Physiology and Pharmacology, 100(6), 542–552. https://doi.org/10.1139/cjpp-2021-0664
Pan, X., Ye, L., Ren, Z., Li, J., Li, B., Pan, L. L., & Sun, J. (2023). Biochanin A ameliorates caerulein-induced acute pancreatitis and associated intestinal injury in mice by inhibiting TLR4 signaling. The Journal of Nutritional Biochemistry, 113, 109229. https://doi.org/10.1016/j.jnutbio.2022.109229
Ren, S., Pan, L., Yang, L., Niu, Z., Wang, L., Gao, Y., Liu, J., Liu, Z., & Pei, H. (2022). Interfering hsa_circ_0073748 alleviates caerulein-induced ductal cell injury in acute pancreatitis by inhibiting miR-132-3p/TRAF3/NF-κB pathway. Cell Cycle, 21(2), 172–186. https://doi.org/10.1080/15384101.2021.2014653
Sun, Y., Shen, W., Hu, S., Lyu, Q., Wang, Q., Wei, T., Zhu, W., & Zhang, J. (2023). METTL3 promotes chemoresistance in small cell lung cancer by inducing mitophagy. Journal of Experimental & Clinical Cancer Research, 42(1), 65. https://doi.org/10.1186/s13046-023-02638-9
Jiang, Y., Zhao, J., Li, R., Liu, Y., Zhou, L., Wang, C., Lv, C., Gao, L., & Cui, D. (2022). CircLRFN5 inhibits the progression of glioblastoma via PRRX2/GCH1 mediated ferroptosis. Journal of Experimental & Clinical Cancer Research, 41(1), 307. https://doi.org/10.1186/s13046-022-02518-8
Neviani, P., Wise, P. M., Murtadha, M., Liu, C. W., Wu, C. H., Jong, A. Y., Seeger, R. C., & Fabbri, M. (2019). Natural killer-derived exosomal miR-186 inhibits neuroblastoma growth and immune escape mechanisms. Cancer Research, 79(6), 1151–1164. https://doi.org/10.1158/0008-5472.can-18-0779
Hu, Z., Chen, G., Zhao, Y., Gao, H., Li, L., Yin, Y., Jiang, J., Wang, L., Mang, Y., Gao, Y., Zhang, S., Ran, J., & Li, L. (2023). Exosome-derived circCCAR1 promotes CD8+ T-cell dysfunction and anti-PD1 resistance in hepatocellular carcinoma. Molecular Cancer, 22(1), 55. https://doi.org/10.1186/s12943-023-01759-1
Dudekula, D. B., Panda, A. C., Grammatikakis, I., De, S., Abdelmohsen, K., & Gorospe, M. (2016). CircInteractome: A web tool for exploring circular RNAs and their interacting proteins and microRNAs. RNA Biology, 13(1), 34–42. https://doi.org/10.1080/15476286.2015.1128065
Li, J. H., Liu, S., Zhou, H., Qu, L. H., & Yang, J. H. (2014). starBase v2.0: Decoding miRNA-ceRNA, miRNA-ncRNA and protein-RNA interaction networks from large-scale CLIP-Seq data. Nucleic Acids Research, 42(Database issue), D92–D97. https://doi.org/10.1093/nar/gkt1248
Yao, B., Zhang, Q., Yang, Z., An, F., Nie, H., Wang, H., Yang, C., Sun, J., Chen, K., Zhou, J., Bai, B., Gu, S., Zhao, W., & Zhan, Q. (2022). CircEZH2/miR-133b/IGF2BP2 aggravates colorectal cancer progression via enhancing the stability of m(6)A-modified CREB1 mRNA. Molecular Cancer, 21(1), 140. https://doi.org/10.1186/s12943-022-01608-7
Gorsky, V. A., Agapov, M. A., Khoreva, M. V., & Leonenko, I. V. (2015). The effect of lornoxicam on TLR2 and TLR4 messenger RNA expression and tumor necrosis factor-α, interleukin-6, and interleukin-8 secretion in patients with systemic complications of acute pancreatitis. Pancreas, 44(5), 824–830. https://doi.org/10.1097/mpa.0000000000000344
Hong, Y. P., Yu, J., Su, Y. R., Mei, F. C., Li, M., Zhao, K. L., Zhao, L., Deng, W. H., Chen, C., & Wang, W. X. (2020). High-fat diet aggravates acute pancreatitis via TLR4-mediated necroptosis and inflammation in rats. Oxidative Medicine and Cellular Longevity, 2020, 8172714. https://doi.org/10.1155/2020/8172714
Pan, L. F., Yu, L., Wang, L. M., He, J. T., Sun, J. L., Wang, X. B., Wang, H., Bai, Z. H., Feng, H., & Pei, H. H. (2018). Augmenter of liver regeneration (ALR) regulates acute pancreatitis via inhibiting HMGB1/TLR4/NF-κB signaling pathway. American Journal of Translational Research, 10(2), 402–410.
Abdelmageed, M. E., Nader, M. A., & Zaghloul, M. S. (2021). Targeting HMGB1/TLR4/NF-κB signaling pathway by protocatechuic acid protects against l-arginine induced acute pancreatitis and multiple organs injury in rats. European Journal of Pharmacology, 906, 174279. https://doi.org/10.1016/j.ejphar.2021.174279
Liu, L. W., Xie, Y., Li, G. Q., Zhang, T., Sui, Y. H., Zhao, Z. J., Zhang, Y. Y., Yang, W. B., Geng, X. L., Xue, D. B., Chen, H., Wang, Y. W., Lu, T. Q., Shang, L. R., Li, Z. B., Li, L., & Sun, B. (2023). Gut microbiota-derived nicotinamide mononucleotide alleviates acute pancreatitis by activating pancreatic SIRT3 signalling. British Journal of Pharmacology, 180(5), 647–666. https://doi.org/10.1111/bph.15980
Sun, Q., Liang, R., Li, M., & Zhou, H. (2022). Circ_UTRN ameliorates caerulein-induced acute pancreatitis in vitro via reducing inflammation and promoting apoptosis through miR-320-3p/PTK2 axis. Journal of Pharmacy and Pharmacology, 74(6), 861–868. https://doi.org/10.1093/jpp/rgab161
Nishita-Hiresha, V., Varsha, R., Jayasuriya, R., & Ramkumar, K. M. (2023). The role of circRNA-miRNA-mRNA interaction network in endothelial dysfunction. Gene, 851, 146950. https://doi.org/10.1016/j.gene.2022.146950
Xu, J., Xu, W., Yang, X., Liu, Z., & Sun, Q. (2021). LncRNA HCG11/miR-579-3p/MDM2 axis modulates malignant biological properties in pancreatic carcinoma via Notch/Hes1 signaling pathway. Aging (Albany NY), 13(12), 16471–16484. https://doi.org/10.18632/aging.203167
Min, X. L., Jia, W. J., Guo, L., Jing, R., Zhao, X. H., Hu, J. Y., Li, X. H., Liu, W., Wang, T., & Dou, X. K. (2024). Brain microvascular endothelial cell-derived exosomes transmitting circ_0000495 promote microglial M1-polarization and endothelial cell injury under hypoxia condition. The FASEB Journal, 38(2), e23387. https://doi.org/10.1096/fj.202301637R
Deng, Q., Huang, J., Yan, J., Mao, E., Chen, H., & Wang, C. (2021). Circ_0001490/miR-579-3p/FSTL1 axis modulates the survival of mycobacteria and the viability, apoptosis and inflammatory response in Mycobacterium tuberculosis-infected macrophages. Tuberculosis (Edinburgh), 131, 102123. https://doi.org/10.1016/j.tube.2021.102123
Jia, J., Cui, Y., Tan, Z., Ma, W., & Jiang, Y. (2020). MicroRNA-579-3p exerts neuroprotective effects against ischemic stroke via anti-inflammation and anti-apoptosis. Neuropsychiatric Disease and Treatment, 16, 1229–1238. https://doi.org/10.2147/ndt.s240698
Wei, B., Wu, Q., Yang, X., Lai, C., Su, Z., & Liang, Z. (2022). Effect of TRAF6 in acute pancreatitis-induced intestinal barrier injury via TLR4/NF-κB signal pathway. Tissue and Cell, 76, 101792. https://doi.org/10.1016/j.tice.2022.101792
Wu, J., Liu, X., Xiao, H., Xu, L., Tang, Z., Wu, Y., & Zhang, X. (2022). Protective effects of HTD4010, a Reg3a/PAP-derived peptide, in a mouse model of hypertriglyceridemic acute pancreatitis: Involvement of TLR4/NF-kappa B. Biochemical and Biophysical Research Communications, 630, 118–124. https://doi.org/10.1016/j.bbrc.2022.09.047
Funding
None.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflicts of interest.
Ethical Approval
The research was granted by the Ethics Committee of Baoan Central Hospital of Shenzhen.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lu, X., Shi, C. & Fan, C. Involvement of circ_0029407 in Caerulein-Evoked Cytotoxicity in Human Pancreatic Cells via the miR-579-3p/TLR4/NF-κB Pathway. Mol Biotechnol (2024). https://doi.org/10.1007/s12033-024-01175-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12033-024-01175-w