Abstract
To investigate the inhibitory effect of hirudin on the cell proliferation of human ovarian cancer A2780 cells by preventing thrombin and its underlying molecular mechanism. Cell Counting Kit-8 (CCK-8) method was used to detect the effect of different concentrations of hirudin and thrombin on the cell proliferation of A2780 cells. PAR-1 wild-type overexpression plasmid was constructed utilizing enzyme digestion identification, and it was transferred to A2780 cells. Sequencing and Western blot were used to detect the changes in PAR-1 protein expression. Western blot detection of PKCα protein phosphorylation in A2780 cells was performed. We also implemented quantitative PCR to detect the mRNA expression levels of epithelial-mesenchymal transition (EMT)-related genes, CDH2, Snail, and Vimentin, in A2780 cells. 1 μg/ml hirudin treatment maximally inhibited the promotion of A2780 cell proliferation by thrombin. Hirudin inhibited the binding of thrombin to the N-terminus of PAR-1, hindered PKCα protein phosphorylation in A2780 cells, and downregulated the mRNA expression levels of CDH2, Snail, and Vimentin. In conclusion, hirudin inhibits the cell proliferation of ovarian cancer A2780 cells, and the underlying mechanism may be through downregulating the transcription level of EMT genes, CDH2, Snail, and Vimentin. This study indicates that hirudin may have a therapeutic potential as an anti-cancer agent for ovarian cancer.
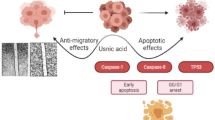
Graphical Abstract





Similar content being viewed by others
Data Availability
All data generated or used during the study appear in the submitted article.
References
Siegel, R. L., Miller, K. D., & Jemal, A. (2020). Cancer statistics, 2020. CA: A Cancer Journal for Clinicians, 70(1), 7–30.
Nguyen, V. H. L., Yue, C., Du, K. Y., Salem, M., O’Brien, J., & Peng, C. (2020). The role of microRNAs in epithelial ovarian cancer metastasis. International Journal of Molecular Sciences, 21(19), 7093.
Achen, G., Dolivet, E., Turck, M., & Fauvet, R. (2020). Incidence et impact de la maladie thrombo-embolique sur la prise en charge du cancer de l’ovaire [Incidence and impact of venous thrombosis in the diagnosis and therapeutic management of ovarian cancer]. Gynecol Obstet Fertil Senol, 48(6), 506–513.
Yanaranop, M. (2017). Role of hypercoagulable state for predictive ovarian malignancy in women with a pelvic mass. Journal of the Medical Association of Thailand, 100(Suppl 1), S148–S156.
Man, Y. N., Wang, Y. N., Hao, J., Liu, X., Liu, C., Zhu, C., & Wu, X. Z. (2015). Pretreatment plasma D-dimer, fibrinogen, and platelet levels significantly impact prognosis in patients with epithelial ovarian cancer independently of venous thromboembolism. International Journal of Gynecological Cancer, 25(1), 24–32.
Huang, Z., Kondoh, E., Visco, Z. R., Baba, T., Matsumura, N., Dolan, E., Whitaker, R. S., Konishi, I., Fujii, S., Berchuck, A., & Murphy, S. K. (2021). Targeting dormant ovarian cancer cells in vitro and in an in vivo mouse model of platinum resistance. Molecular Cancer Therapeutics, 20(1), 85–95.
Song, J. S., Kang, C. M., Park, C. K., & Yoon, H. K. (2013). Thrombin induces epithelial-mesenchymal transition via PAR-1, PKC, and ERK1/2 pathways in A549 cells. Experimental Lung Research, 39(8), 336–348.
Kim, K. Y., Choi, K. C., Auersperg, N., & Leung, P. C. (2006). Mechanism of gonadotropin-releasing hormone (GnRH)-I and -II-induced cell growth inhibition in ovarian cancer cells: Role of the GnRH-I receptor and protein kinase C pathway. Endocrine-Related Cancer, 13(1), 211–220.
Al-Alem, L. F., McCord, L. A., Southard, R. C., Kilgore, M. W., & Curry, T. E., Jr. (2013). Activation of the PKC pathway stimulates ovarian cancer cell proliferation, migration, and expression of MMP7 and MMP10. Biology of Reproduction, 89(3), 73.
Hong, L., Yingxue, Z., & Linxiu, W. (2023). To explore the mechanism of hirudin in the treatment of gouty arthritis based on TGF-β1/Smads signaling pathway. Chinese Herbal Medicine, 46(7), 1783–1787.
Zhao, L. (2015). Hirudin inhibits cell growth via ERK/MAPK signaling in human glioma. International Journal of Clinical and Experimental Medicine, 8(11), 20983–20987.
Lu, Q., Lv, M., Xu, E., Shao, F., Feng, Y., Yang, J., & Shi, L. (2015). Recombinant hirudin suppresses the viability, adhesion, migration, and invasion of Hep-2 human laryngeal cancer cells. Oncology Reports, 33(3), 1358–1364.
Zhong, Y. C., Zhang, T., Di, W., & Li, W. P. (2013). Thrombin promotes epithelial ovarian cancer cell invasion by inducing epithelial-mesenchymal transition. Journal of Gynecologic Oncology, 24(3), 265–272.
Pang, X., Zhang, Y., Peng, Z., Shi, X., Han, J., & Xing, Y. (2020). Hirudin reduces nephropathy microangiopathy in STZ-induced diabetes rats by inhibiting endothelial cell migration and angiogenesis. Life Sciences, 255, 117779.
Han, X., Nieman, M. T., & Kerlin, B. A. (2020). Protease-activated receptors: An illustrated review. Res Pract Thromb Haemost., 5(1), 17–26.
Steinberg, S. F. (2005). The cardiovascular actions of protease-activated receptors. Molecular Pharmacology, 67(1), 2–11.
Li, Q. J., Wu, Z. L., Wang, J., Jiang, J., & Lin, B. (2023). An EMT-based gene signature enhances the clinical understanding and prognostic prediction of patients with ovarian cancers. J Ovarian Res., 16(1), 51.
Li, H., Gao, Y., & Ren, C. (2021). Focal adhesion kinase inhibitor BI 853520 inhibits cell proliferation, migration and EMT process through PI3K/AKT/mTOR signaling pathway in ovarian cancer. Discov Oncol., 12(1), 29.
Nuti, S. V., Mor, G., Li, P., & Yin, G. (2014). TWIST and ovarian cancer stem cells: Implications for chemoresistance and metastasis. Oncotarget, 5(17), 7260–7271.
Swier, N., & Versteeg, H. H. (2017). Reciprocal links between venous thromboembolism, coagulation factors and ovarian cancer progression. Thrombosis Research, 150, 8–18.
Falanga, A., Schieppati, F., & Russo, D. (2015). Cancer tissue procoagulant mechanisms and the hypercoagulable state of patients with cancer. Seminars in Thrombosis and Hemostasis, 41(7), 756–764.
Franchini, M., & Mannucci, P. M. (2012). Thrombin and cancer: From molecular basis to therapeutic implications. Seminars in Thrombosis and Hemostasis, 38(1), 95–101.
Reddel, C. J., Allen, J. D., Ehteda, A., Taylor, R., Curnow, J. L., Kritharides, L., & Robertson, G. (2017). Increased thrombin generation in a mouse model of cancer cachexia is partially interleukin-6 dependent. Journal of Thrombosis and Haemostasis, 15(3), 477–486.
Lima, L. G., Ham, S., Shin, H., Chai, E. P. Z., Lek, E. S. H., Lobb, R. J., Müller, A. F., Mathivanan, S., Yeo, B., Choi, Y., Parker, B. S., & Möller, A. (2021). Tumor microenvironmental cytokines bound to cancer exosomes determine uptake by cytokine receptor-expressing cells and biodistribution. Nature Communications, 12(1), 3543.
Yu, J. L., May, L., Lhotak, V., Shahrzad, S., Shirasawa, S., Weitz, J. I., Coomber, B. L., Mackman, N., & Rak, J. W. (2005). Oncogenic events regulate tissue factor expression in colorectal cancer cells: Implications for tumor progression and angiogenesis. Blood, 105(4), 1734–1741.
Khorana, A. A., Francis, C. W., Menzies, K. E., Wang, J. G., Hyrien, O., Hathcock, J., Mackman, N., & Taubman, M. B. (2008). Plasma tissue factor may be predictive of venous thromboembolism in pancreatic cancer. Journal of Thrombosis and Haemostasis, 6(11), 1983–1985.
Zhu, J., Pan, X., Lin, B., Lin, G., Pradhan, R., Long, F., & Yin, G. (2019). The effect of hirudin on antagonisting thrombin induced apoptosis of human microvascular endothelial cells1. Acta cirurgica Brasileira, 34(1), e20190010000006.
Sun, Z., Zhao, Z., Zhao Sheng, S. Y., Zhao, Z., Gao, C., Li, J., & Liu, X. (2009). Recombinant hirudin treatment modulates aquaporin-4 and aquaporin-9 expression after intracerebral hemorrhage in vivo. Molecular Biology Reports, 36(5), 1119–1127.
Lu, Q., Lv, M., Xu, E., Shao, F., Feng, Y., Yang, J., & Shi, L. (2015). Recombinant hirudin suppresses the viability, adhesion, migration and invasion of Hep-2 human laryngeal cancer cells. Oncology Reports, 33(3), 1358–1364.
Ebrahimi, S., Rahmani, F., Behnam-Rassouli, R., Hoseinkhani, F., Parizadeh, M. R., Keramati, M. R., Khazaie, M., Avan, A., & Hassanian, S. M. (2017). Proinflammatory signaling functions of thrombin in cancer. Journal of Cellular Physiology, 232(9), 2323–2329.
Wojtukiewicz, M. Z., Hempel, D., Sierko, E., Tucker, S. C., & Honn, K. V. (2019). Endothelial protein C receptor (EPCR), protease activated receptor-1 (PAR-1) and their interplay in cancer growth and metastatic dissemination. Cancers (Basel), 11(1), 51.
Anderluh, M., & Dolenc, M. S. (2002). Thrombin receptor antagonists; recent advances in PAR-1 antagonist development. Current Medicinal Chemistry, 9(13), 1229–1250.
Wang, T., Jiao, J., Zhang, H., Zhou, W., Li, Z., Han, S., Wang, J., Yang, X., Huang, Q., Zhipeng, W., Yan, W., & Xiao, J. (2017). TGF-β induced PAR-1 expression promotes tumor progression and osteoclast differentiation in giant cell tumor of bone. International Journal of Cancer, 141(8), 1630–1642.
Wang, Q., Yang, H., Zhuo, Q., Xu, Y., & Zhang, P. (2018). Knockdown of EPCR inhibits the proliferation and migration of human gastric cancer cells via the ERK1/2 pathway in a PAR-1-dependent manner. Oncology Reports, 39(4), 1843–1852.
Funding
This work was supported by the Traditional Chinese Medicine Science and Technology Program Project of Zhejiang Province (No. 2022ZA140).
Author information
Authors and Affiliations
Contributions
JK and JD dedicated to the study concepts, study design and responsible for the integrity of the entire study; LG and LN were involved in the data acquisition, data analysis and statistical analysis; TS carried out the literature research and manuscript preparation and manuscript writing. All authors have reviewed and approved this article.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interests.
Ethical Approval
The current study was conducted with the approval of the Ethical Committees of Hangzhou Cancer Hospital.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kou, J., Gao, L., Ni, L. et al. Mechanism of Hirudin-Mediated Inhibition of Proliferation in Ovarian Cancer Cells. Mol Biotechnol 66, 1062–1070 (2024). https://doi.org/10.1007/s12033-023-01003-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12033-023-01003-7