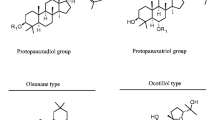
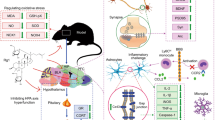
Abstract
There is a need for an efficient and long-lasting treatment due to the population's increasing prevalence of neurodegenerative disorders. In an effort to generate fresh ideas and create novel therapeutic medications, scientists have recently started to investigate the biological functions of compounds derived from plants and herbs. Ginseng, famous Chinese herbal medicine, has therapeutic value by virtue of its compounds ginsenosides or panaxosides, which are triterpene saponins and steroid glycosides. Research revealed positive impacts on ameliorating various disease conditions and found it as a possible drug candidate. Several neuroprotection mechanisms followed by this compound are inhibition of cell apoptosis, oxidative stress, inflammatory, and tumor activity. It has been demonstrated that controlling these mechanisms enhances cognitive performance and safeguards the brain against neurodegenerative disorders. The main objective of this review is to give a description of the most recent studies on ginsenoside’s possible therapeutic application in the treatment of neurodegenerative diseases. Using organic compounds like ginseng and its various components may create new avenues for innovative treatment approaches development for neurological diseases. However, further research is necessary to confirm the stability and effectiveness of ginsenosides for neurodegenerative disease.
Graphical Abstract






Similar content being viewed by others
Data Availability
The authors confirm that the data supporting the findings of this study are available within the article.
References
Walia, S., Kumar, P., Kumar, D., & Kumar, R. (2023). A preliminary study on suitability of growing ginseng (Panax ginseng Meyer) in western Himalayan region. Plant, Soil and Environment, 69(2), 71–80. https://doi.org/10.17221/288/2022-PSE
Amir, M., Vohra, M., Raj, R. G., Osoro, I., & Sharma, A. (2023). Adaptogenic herbs: A natural way to improve athletic performance. Health Sciences Review. https://doi.org/10.1016/j.hsr.2023.100092
Balusamy, S. R., et al. (2023). A comprehensive and systemic review of ginseng-based nanomaterials: Synthesis, targeted delivery, and biomedical applications. Medicinal Research Reviews. https://doi.org/10.1002/med.21953
C.-Z. Wang, S. Anderson, W. DU, T.-C. He, and C.-S. Yuan, (2016) “Red ginseng and cancer treatment.,” Chin J Nat Med, vol. 14, no. 1, pp. 7–16
Zhang, H., et al. (2020). Characteristics of panax ginseng cultivars in Korea and China. Molecules. https://doi.org/10.3390/molecules25112635
Sahiner, M., Yilmaz, A. S., Gungor, B., & Sahiner, N. (2023). A review on phyto-therapeutic approaches in alzheimer’s disease. J Funct Biomater, 14(1), 50. https://doi.org/10.3390/jfb14010050
Babazadeh, A., Vahed, F. M., Liu, Q., Siddiqui, S. A., Kharazmi, M. S., & Jafari, S. M. (2023). Natural bioactive molecules as neuromedicines for the treatment/prevention of neurodegenerative diseases. ACS Omega, 8(4), 3667–3683. https://doi.org/10.1021/acsomega.2c06098
Sun, X.-F., & Pradeep Singh, S. (2023). Network pharmacology integrated molecular docking demonstrates the therapeutic mode of Panax ginseng against ovarian cancer. Tropical Journal of Pharmaceutical Research, 22(3), 589–596. https://doi.org/10.4314/tjpr.v22i3.16
Baik, I.-H., Kim, K.-H., & Lee, K.-A. (2021). Antioxidant, anti-inflammatory and antithrombotic effects of ginsenoside compound K enriched extract derived from ginseng sprouts. Molecules, 26(13), 4102. https://doi.org/10.3390/molecules26134102
“Calories in Ginseng.” https://www.nutritionix.com/food/ginseng (accessed Apr. 07, 2023).
Guo, M., Shao, S., Wang, D., Zhao, D., & Wang, M. (2021). Recent progress in polysaccharides from Panax ginseng C. A. Meyer. Food & Function, 12(2), 494–518. https://doi.org/10.1039/D0FO01896A
Lee, J., et al. (2017). Mass spectrometry based profiling and imaging of various ginsenosides from panax ginseng roots at different ages. International Journal of Molecular Sciences. https://doi.org/10.3390/IJMS18061114
Lee, I.-S., Kang, K. S., & Kim, S.-Y. (2019). Panax ginseng pharmacopuncture: current status of the research and future challenges. Biomolecules, 10(1), 33. https://doi.org/10.3390/biom10010033
Karmazyn, M., & Gan, X. T. (2021). Chemical components of ginseng, their biotransformation products and their potential as treatment of hypertension. Molecular and Cellular Biochemistry, 476(1), 333–347. https://doi.org/10.1007/s11010-020-03910-8
S. Khan, A. Tosun, and Y. S. Kim, “Ginsenosides as Food Supplements and Their Potential Role in Immunological and Neurodegenerative Disorders,” in Bioactive Nutraceuticals and Dietary Supplements in Neurological and Brain Disease, Elsevier, 2015, pp. 303–309. doi: https://doi.org/10.1016/B978-0-12-411462-3.00031-X.
Lee, D. G., Lee, J. S., Kim, K.-T., Kim, H. Y., & Lee, S. (2019). Analysis of major ginsenosides in various ginseng samples. Journal of Applied Biological Chemistry, 62(1), 87–91. https://doi.org/10.3839/jabc.2019.013
Chen, W., Balan, P., & Popovich, D. G. (2020). Comparison of ginsenoside components of various tissues of new zealand forest-grown asian ginseng (Panax ginseng) and american ginseng (panax quinquefolium l.). Biomolecules. https://doi.org/10.3390/biom10030372
Jia, X. H., et al. (2013). Comparative studies of saponins in 1–3-year-old main roots, fibrous roots, and rhizomes of Panax notoginseng, and identification of different parts and growth-year samples. Journal of Natural Medicines, 67(2), 339–349. https://doi.org/10.1007/s11418-012-0691-6
T. Hong Van Le et al., “Ginseng Saponins in Different Parts of Panax vietnamensis,” 2015.
P. Mikulska et al., “Ashwagandha (Withania somnifera)—Current research on the health-promoting activities: a narrative review,” Pharmaceutics 2023, vol. 15, no. 4, p. 1057, 2023, doi: https://doi.org/10.3390/PHARMACEUTICS15041057.
Mohsin, M. M., Hanif, M. A., Ayub, M. A., & Dharmadasa, R. M. (2022). Ginseng (pp. 331–340). Amsterdam: Medicinal Plants of South Asia Novel Sources for Drug Discovery. Elsevier.
Lee, J.-H., Ahn, N.-H., Choi, S.-B., Kwon, Y., & Yang, S.-H. (2021). Natural products targeting amyloid beta in Alzheimer’s Disease. International Journal of Molecular Sciences, 22(5), 2341. https://doi.org/10.3390/ijms22052341
Abduljawad, A. A., et al. (2022). Alzheimer’s disease as a major public health concern: role of dietary saponins in mitigating neurodegenerative disorders and their underlying mechanisms. Molecules, 27(20), 6804. https://doi.org/10.3390/molecules27206804
P. T. Lum, M. Sekar, S. H. Gan, S. R. Bonam, and Mohd. F. Shaikh, “Protective effect of natural products against huntington’s disease an overview of scientific evidence and understanding their mechanism of action,” ACS Chem Neuroscience, 2021, doi: https://doi.org/10.1021/acschemneuro.0c00824.
Zha, Z., Liu, S., Liu, Y., Li, C., & Wang, L. (2022). Potential utility of natural products against oxidative stress in animal models of multiple sclerosis. Antioxidants, 11(8), 1495. https://doi.org/10.3390/antiox11081495
He, et al. (2019). Recent advances in biotransformation of saponins. Molecules, 24(13), 2365. https://doi.org/10.3390/molecules24132365
Hou, M., Wang, R., Zhao, S., & Wang, Z. (2021). Ginsenosides in Panax genus and their biosynthesis. Acta Pharm Sin B, 11(7), 1813–1834. https://doi.org/10.1016/j.apsb.2020.12.017
W. Chen, P. Balan, and D. G. Popovich, “Comparison of the ginsenoside composition of Asian ginseng (Panax ginseng) and American ginseng (Panax quinquefolius L.) and their transformation pathways,” 2019, pp. 161–195. doi: https://doi.org/10.1016/B978-0-12-817901-7.00006-X.
Sun, Y., et al. (2020). Synthesis and structure-activity relationship of pyxinol derivatives as novel anti-inflammatory agents. ACS Medicinal Chemistry Letters, 11(4), 457–463. https://doi.org/10.1021/acsmedchemlett.9b00562
Lu, C., et al. (2018). The protective effect of 20(S)-protopanaxadiol (PPD) against chronic sleep deprivation (CSD)-induced memory impairments in mice. Brain Research Bulletin, 137, 249–256. https://doi.org/10.1016/j.brainresbull.2017.12.012
Zarneshan, S. N., Fakhri, S., & Khan, H. (2022). Targeting Akt/CREB/BDNF signaling pathway by ginsenosides in neurodegenerative diseases: A mechanistic approach. Pharmacological Research. https://doi.org/10.1016/j.phrs.2022.106099
Ahmed, T., et al. (2016). Ginsenoside Rb1 as a neuroprotective agent: A review. Brain Research Bulletin, 125, 30–43. https://doi.org/10.1016/j.brainresbull.2016.04.002
Gao, H., et al. (2020). Ginsenoside Rb1 exerts anti-inflammatory effects in vitro and in vivo by modulating toll-like receptor 4 dimerization and NF-kB/MAPKs signaling pathways. Phytomedicine. https://doi.org/10.1016/j.phymed.2020.153197
Kim, J. H., Yi, Y.-S., Kim, M.-Y., & Cho, J. Y. (2017). Role of ginsenosides, the main active components of Panax ginseng, in inflammatory responses and diseases. Journal of Ginseng Research, 41(4), 435–443. https://doi.org/10.1016/j.jgr.2016.08.004
Qu, B., Cao, T., Wang, M., Wang, S., Li, W., & Li, H. (2022). Ginsenosides Rd monomer inhibits proinflammatory cytokines production and alleviates DSS-colitis by NF-κB and P38MAPK pathways in mice. Immunopharmacology and Immunotoxicology, 44(1), 110–118. https://doi.org/10.1080/08923973.2021.2012482
Moratilla-Rivera, I., Sánchez, M., Valdés-González, J. A., & Gómez-Serranillos, M. P. (2023). Natural products as modulators of Nrf2 signaling pathway in neuroprotection. International Journal of Molecular Sciences, 24(4), 3748. https://doi.org/10.3390/IJMS24043748
Y.-J. Ji, “Heat Treatment Enhances the Neuroprotective Effects of Crude Ginseng Saponin by Increasing Minor Ginsenosides,” 2023, doi: https://doi.org/10.21203/rs.3.rs-2532316/v1.
Hou, J., Xue, J., Wang, Z., & Li, W. (2018). Ginsenoside Rg3 and Rh2 protect trimethyltin-induced neurotoxicity via prevention on neuronal apoptosis and neuroinflammation. Phytotherapy Research, 32(12), 2531–2540. https://doi.org/10.1002/ptr.6193
Wang, Z., Zhang, Z., Liu, J., Guo, M., & Li, H. (2023). Panax Ginseng in the treatment of Alzheimer’s disease and vascular dementia. Journal of Ginseng Research. https://doi.org/10.1016/j.jgr.2023.03.001
Xu, C., et al. (2022). Effects of G-Rh2 on mast cell-mediated anaphylaxis via AKT-Nrf2/NF-κB and MAPK-Nrf2/NF-κB pathways. Journal of Ginseng Research, 46(4), 550–560. https://doi.org/10.1016/j.jgr.2021.10.001
“The Power of Natural Chinese Medicine, Ginger and Ginseng Root in an Organic Life”, doi: https://doi.org/10.5829/idosi.mejsr.2019.64.71.
B. de Oliveira Zanuso, A. R. de Oliveira dos Santos, V. F. B. Miola, L. M. Guissoni Campos, C. S. G. Spilla, and S. M. Barbalho, “Panax ginseng and aging related disorders: A systematic review,” Experimental Gerontology, 2022, doi: https://doi.org/10.1016/j.exger.2022.111731.
Wang, Z.-Y., et al. (2021). Traditional Chinese medicine compounds regulate autophagy for treating neurodegenerative disease: A mechanism review. Biomedicine & Pharmacotherapy. https://doi.org/10.1016/j.biopha.2020.110968
Lee, J.-W., et al. (2021). The Role of Microglia in the Development of Neurodegenerative Diseases. Biomedicines, 9(10), 1449. https://doi.org/10.3390/biomedicines9101449
Liu, S., et al. (2018). Intravenous immunoglobulin ameliorates motor and cognitive deficits and neuropathology in R6/2 mouse model of Huntington’s disease by decreasing mutant huntingtin protein level and normalizing NF-κB signaling pathway. Brain Research, 1697, 21–33. https://doi.org/10.1016/j.brainres.2018.06.009
Lee, S.-Y., Jeong, J.-J., Eun, S.-H., & Kim, D.-H. (2015). Anti-inflammatory effects of ginsenoside Rg1 and its metabolites ginsenoside Rh1 and 20(S)-protopanaxatriol in mice with TNBS-induced colitis. European Journal of Pharmacology, 762, 333–343. https://doi.org/10.1016/j.ejphar.2015.06.011
Cui, J., Shan, R., Cao, Y., Zhou, Y., Liu, C., & Fan, Y. (2021). Protective effects of ginsenoside Rg2 against memory impairment and neuronal death induced by Aβ25-35 in rats. Journal of Ethnopharmacology. https://doi.org/10.1016/J.JEP.2020.113466
Lu, Q., Li, R., Yang, Y., Zhang, Y., Zhao, Q., & Li, J. (2022). Ingredients with anti-inflammatory effect from medicine food homology plants. Food Chemistry. https://doi.org/10.1016/J.FOODCHEM.2021.130610
Kim, M., Mok, H., Yeo, W.-S., Ahn, J.-H., & Choi, Y. K. (2021). Role of ginseng in the neurovascular unit of neuroinflammatory diseases focused on the blood-brain barrier. Journal of Ginseng Research, 45(5), 599–609. https://doi.org/10.1016/j.jgr.2021.02.003
Tam, D. N. H., et al. (2018). Ginsenoside Rh1: A systematic review of its pharmacological properties. Planta Medica, 84(3), 139–152. https://doi.org/10.1055/S-0043-124087/ID/RB0467-2
“2017 Alzheimer’s disease facts and figures,” Alzheimer’s & Dementia, vol. 13, no. 4, pp. 325–373, 2017, doi: https://doi.org/10.1016/j.jalz.2017.02.001.
Wu, J., et al. (2022). New insights into the role and mechanisms of ginsenoside Rg1 in the management of Alzheimer’s disease. Biomedicine & Pharmacotherapy. https://doi.org/10.1016/j.biopha.2022.113207
Abeysinghe, A. A. D. T., Deshapriya, R. D. U. S., & Udawatte, C. (2020). Alzheimer’s disease; a review of the pathophysiological basis and therapeutic interventions. Life Sciences. https://doi.org/10.1016/j.lfs.2020.117996
Liang, H.-Y., et al. (2021). Preclinical systematic review of ginsenoside Rg1 for cognitive impairment in Alzheimer’s disease. Aging, 13(5), 7549–7569. https://doi.org/10.18632/aging.202619
Chen, F., Eckman, E. A., Eckman, C. B., Chen, F., Eckman, E. A., & Eckman, C. B. (2006). Reductions in levels of the Alzheimer’s amyloid β peptide after oral administration of ginsenosides. The FASEB Journal, 20(8), 1269–1271. https://doi.org/10.1096/fj.05-5530fje
Bhat, B. A., et al. (2022). Natural therapeutics in aid of treating alzheimer’s disease: a green gateway toward ending quest for treating neurological disorders. Frontiers in Neuroscience. https://doi.org/10.3389/fnins.2022.884345
Zhang, H., et al. (2021). Ginsenoside Rg1 alleviates Aβ deposition by inhibiting NADPH oxidase 2 activation in APP/PS1 mice. Journal of Ginseng Research, 45(6), 665–675. https://doi.org/10.1016/j.jgr.2021.03.003
Liu, Y., Gao, Y., Li, K.-X., & Xue, W. (2019). Pharmacokinetics and acetylcholine releasing effects of ginsenoside Rg1 in hippocampus of beta-amyloid model rats. Journal of Asian Natural Products Research, 21(8), 772–781. https://doi.org/10.1080/10286020.2018.1540596
Guo, Y., et al. (2021). Ginsenoside Rg1 improves cognitive capability and affects the microbiota of large intestine of tree shrew model for Alzheimer’s disease. Molecular Medicine Reports, 23(4), 291. https://doi.org/10.3892/mmr.2021.11931
Zhang, Y., et al. (2021). Ginsenoside Rg1 alleviates lipopolysaccharide-induced neuronal damage by inhibiting NLRP1 inflammasomes in HT22 cells. Experimental and Therapeutic Medicine. https://doi.org/10.3892/etm.2021.10214
Feng, H., Xue, M., Deng, H., Cheng, S., Hu, Y., & Zhou, C. (2022). Ginsenoside and its therapeutic potential for cognitive impairment. Biomolecules, 12(9), 1310. https://doi.org/10.3390/biom12091310
Pačesová, A., et al. (2022). Age-related metabolic and neurodegenerative changes in SAMP8 mice. Aging, 14(18), 7300–7327. https://doi.org/10.18632/aging.204284
Wang, Y., et al. (2018). Ginsenoside Rb1 inhibit apoptosis in rat model of Alzheimer’s disease induced by Aβ1-40. Am J Transl Res, 10(3), 796–805.
van der Flier, W. M., et al. (2018). Vascular cognitive impairment. Nature Reviews. Disease Primers. https://doi.org/10.1038/nrdp.2018.3
Dinh, Q. N., Vinh, A., Arumugam, T. V., Drummond, G. R., & Sobey, C. G. (2021). G protein-coupled estrogen receptor 1: a novel target to treat cardiovascular disease in a sex-specific manner? British Jounal of Pharmacology, 178(19), 3849–3863. https://doi.org/10.1111/bph.15521
Zeng, X., et al. (2018). The effects of ginsenoside compound K against epilepsy by enhancing the γ-aminobutyric acid signaling pathway. Frontiers in Pharmacology. https://doi.org/10.3389/fphar.2018.01020
Liu, H., Deng, H., Zhao, Y., Li, C., & Liang, Y. (2018). LncRNA XIST/miR-34a axis modulates the cell proliferation and tumor growth of thyroid cancer through MET-PI3K-AKT signaling. Journal of Experimental & Clinical Cancer Research, 37(1), 279. https://doi.org/10.1186/s13046-018-0950-9
Zong, W., et al. (2019). Ginsenoside compound K attenuates cognitive deficits in vascular dementia rats by reducing the Aβ deposition. Journal of Pharmacological Sciences, 139(3), 223–230. https://doi.org/10.1016/j.jphs.2019.01.013
Emamzadeh, F. N., & Surguchov, A. (2018). Parkinson’s disease: biomarkers, treatment, and risk factors. Frontiers in Neuroscience. https://doi.org/10.3389/fnins.2018.00612
Zhang, Y., et al. (2017). Ginsenoside Rg1 protects against neuronal degeneration induced by chronic dexamethasone treatment by inhibiting NLRP-1 inflammasomes in mice. International Journal of Molecular Medicine, 40(4), 1134–1142. https://doi.org/10.3892/ijmm.2017.3092
Li, X., et al. (2022). Ginsenoside Rg1 alleviates learning and memory impairments and Aβ disposition through inhibiting NLRP1 inflammasome and autophagy dysfunction in APP/PS1 mice. Molecular Medicine Reports, 27(1), 6. https://doi.org/10.3892/mmr.2022.12893
Marchetti, B. (2018). Wnt/β-catenin signaling pathway governs a full program for dopaminergic neuron survival, neurorescue and regeneration in the MPTP Mouse Model of Parkinson’s Disease. International Journal of Molecular Sciences, 19(12), 3743. https://doi.org/10.3390/ijms19123743
Ardah, M. T., et al. (2015). Ginsenoside Rb1 inhibits fibrillation and toxicity of alpha-synuclein and disaggregates preformed fibrils. Neurobiology of Diseases, 74, 89–101. https://doi.org/10.1016/j.nbd.2014.11.007
Tang, D., Chen, X., Kang, R., & Kroemer, G. (2021). Ferroptosis: Molecular mechanisms and health implications. Cell Research. https://doi.org/10.1038/s41422-020-00441-1
Hirschhorn, T., & Stockwell, B. R. (2019). The development of the concept of ferroptosis. Free Radical Biology & Medicine, 133, 130–143. https://doi.org/10.1016/j.freeradbiomed.2018.09.043
Chen, Y., Li, Y.-Y., Wang, S., Zhou, T., Chen, N., & Yuan, Y. (2022). Ginsenoside Rg1 plays a neuroprotective role in regulating the iron-regulated proteins and against lipid peroxidation in oligodendrocytes. Neurochemical Research, 47(6), 1721–1735. https://doi.org/10.1007/s11064-022-03564-6
Hauser, R. M., Henshall, D. C., & Lubin, F. D. (2018). The epigenetics of epilepsy and its progression. The Neuroscientist, 24(2), 186–200. https://doi.org/10.1177/1073858417705840
Singh, A., & Trevick, S. (2016). The Epidemiology of Global Epilepsy. Neurologic Clinics, 34(4), 837–847. https://doi.org/10.1016/j.ncl.2016.06.015
Shi, Y., Miao, W., Teng, J., & Zhang, L. (2018). Ginsenoside Rb1 protects the brain from damage induced by epileptic seizure via Nrf2/ARE signaling. Cellular Physiology and Biochemistry, 45(1), 212–225. https://doi.org/10.1159/000486768
Hinton, T., & Johnston, G. A. R. (2018). “GABA, The Major Inhibitory Neurotransmitter in the Brain”, in Reference Module in Biomedical Sciences. Elsevier. https://doi.org/10.1016/B978-0-12-801238-3.96594-2
Walter, C., et al. (2016). Activation of AMPK-induced autophagy ameliorates Huntington disease pathology in vitro. Neuropharmacology, 108, 24–38. https://doi.org/10.1016/j.neuropharm.2016.04.041
Rué, L., et al. (2016). Targeting CAG repeat RNAs reduces Huntington’s disease phenotype independently of huntingtin levels. Journal of Clinical Investigation, 126(11), 4319–4330. https://doi.org/10.1172/JCI83185
Lee, M. J., et al. (2021). Rg3-enriched Korean Red Ginseng extract inhibits blood-brain barrier disruption in an animal model of multiple sclerosis by modulating expression of NADPH oxidase 2 and 4. Journal of Ginseng Research, 45(3), 433–441. https://doi.org/10.1016/j.jgr.2020.09.001
Jang, M., & Cho, I.-H. (2016). Sulforaphane ameliorates 3-nitropropionic acid-induced striatal toxicity by activating the Keap1-Nrf2-ARE pathway and inhibiting the MAPKs and NF-κB pathways. Molecular Neurobiology, 53(4), 2619–2635. https://doi.org/10.1007/s12035-015-9230-2
Lee, M., Ban, J.-J., Won, B. H., Im, W., & Kim, M. (2021). Therapeutic potential of ginsenoside Rg3 and Rf for Huntington’s disease. In Vitro Cellular and Developmental Biology. Animal, 57(6), 641–648. https://doi.org/10.1007/s11626-021-00595-1
X. Yang, S. Feng Chu, Z. zhen Wang, F. fang Li, Y. he Yuan, and N. hong Chen, “Ginsenoside Rg1 exerts neuroprotective effects in 3-nitropronpionic acid-induced mouse model of Huntington’s disease via suppressing MAPKs and NF-κB pathways in the striatum,” Acta Pharmacol Sin, vol. 42, no. 9, pp. 1409–1421, 2021, doi: https://doi.org/10.1038/s41401-020-00558-4.
Danikowski, K. M., Jayaraman, S., & Prabhakar, B. S. (2017). Regulatory T cells in multiple sclerosis and myasthenia gravis. Journal of Neuroinflammation, 14(1), 117. https://doi.org/10.1186/s12974-017-0892-8
Burrows, D. J., et al. (2019). Animal models of multiple sclerosis: From rodents to zebrafish. Multiple Sclerosis Journal. https://doi.org/10.1177/1352458518805246
Zhang, X., Liu, X., Hu, G., Zhang, G., Zhao, G., & Shi, M. (2020). Ginsenoside Rd attenuates blood-brain barrier damage by suppressing proteasome-mediated signaling after transient forebrain ischemia. NeuroReport, 31(6), 466–472. https://doi.org/10.1097/WNR.0000000000001426
Wang, X., et al. (2015). Ginsenoside Rg3 antagonizes adriamycin-induced cardiotoxicity by improving endothelial dysfunction from oxidative stress via upregulating the Nrf2-ARE pathway through the activation of akt. Phytomedicine, 22(10), 875–884. https://doi.org/10.1016/j.phymed.2015.06.010
Jin, B., Zhang, C., Geng, Y., & Liu, M. (2020). Therapeutic effect of ginsenoside rd on experimental autoimmune encephalomyelitis model mice: regulation of inflammation and Treg/Th17 cell balance. Mediators of Inflammation, 2020, 1–12. https://doi.org/10.1155/2020/8827527
Wang, H. Q., Wang, Z. Z., & Chen, N. H. (2021). The receptor hypothesis and the pathogenesis of depression: Genetic bases and biological correlates. Pharmacological Research. https://doi.org/10.1016/j.phrs.2021.105542
Lou, Y.-X., et al. (2020). The protective effect of ginsenoside Rg1 on depression may benefit from the gap junction function in hippocampal astrocytes. European Journal of Pharmacology. https://doi.org/10.1016/j.ejphar.2020.173309
Huang, D., Li, C., Zhang, W., Qin, J., Jiang, W., & Hu, C. (2019). Dysfunction of astrocytic connexins 30 and 43 in the medial prefrontal cortex and hippocampus mediates depressive-like behaviours. Behavioural Brain Research. https://doi.org/10.1016/j.bbr.2019.111950
Wang, H.-Q., et al. (2021). Novel antidepressant mechanism of ginsenoside Rg1: Regulating biosynthesis and degradation of connexin43. Journal of Ethnopharmacology. https://doi.org/10.1016/j.jep.2021.114212
Wang, H., et al. (2021). Ginsenoside Rg1 ameliorates neuroinflammation via suppression of Connexin43 ubiquitination to attenuate depression. Frontiers in Pharmacology. https://doi.org/10.3389/fphar.2021.709019
Ghaeminia, M., Rajkumar, R., Koh, H.-L., Dawe, G. S., & Tan, C. H. (2018). Ginsenoside Rg1 modulates medial prefrontal cortical firing and suppresses the hippocampo-medial prefrontal cortical long-term potentiation. Journal of Ginseng Research, 42(3), 298–303. https://doi.org/10.1016/j.jgr.2017.03.010
Ou, L., Lin, S., Song, B., Liu, J., Lai, R., & Shao, L. (2017). The mechanisms of graphene-based materials-induced programmed cell death: A review of apoptosis, autophagy, and programmed necrosis. International Journal of Nanomedicine, 12, 6633–6646. https://doi.org/10.2147/IJN.S140526
Bates, G. P., et al. (2015). Huntington disease. Nature Reviews Disease Primers. https://doi.org/10.1038/nrdp.2015.5
Seo, J. Y., Ju, S. H., Oh, J., Lee, S. K., & Kim, J. S. (2016). Neuroprotective and cognition-enhancing effects of compound k isolated from red ginseng. Journal of Agriculture and Food Chemistry, 64(14), 2855–2864. https://doi.org/10.1021/acs.jafc.5b05789
Acknowledgements
We appreciate all of the help and advice we have received from the Delhi Technological University in Delhi, India.
Funding
The authors affirm that they did not accept any money, grants, or other assistance for the creation of this manuscript.
Author information
Authors and Affiliations
Contributions
Each author has contributed equally. The final document has been read by all writers.
Corresponding author
Ethics declarations
Conflict of interest
The authors affirm that they have no competing interests with this work. There are no material financial or non-financial interests of the authors to report.
Research Involving Human Participants and/or Animals
No.
Informed Consent
Yes, we are aware of every detail of our involvement.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Manju, Bharadvaja, N. Exploring the Potential Therapeutic Approach Using Ginsenosides for the Management of Neurodegenerative Disorders. Mol Biotechnol (2023). https://doi.org/10.1007/s12033-023-00783-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12033-023-00783-2