Abstract
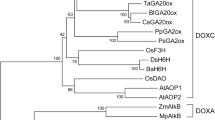
Gibberellins (GAs; tetracyclic di-terpenoid carboxylic acids) are endogenous plant growth regulators responsible for stimulating plant growth and development from seed germination to plant maturity. In potato (Solanum tuberosum L.), GA levels are known to be crucial in the complex process of tuberization. Gibberellin 2-oxidases (GA2oxs) inactivate bioactive GAs during stolon swelling and early stages of tuberization as evident from the predominant expression of a member of this gene family namely GA2ox1. We isolated and characterized a 1105-bp cDNA clone encoding a 340-aa GA2ox1 form, designated St-GA2ox1, using total RNA from growing tuber of a potato (Solanum tuberosum L.) cultivar, Kufri Chipsona-1 (KC-1) based on RT-PCR approach. A total of 26 GA2ox sequences were also retrieved from potato genome database and analysed. Multiple sequence alignment revealed sequence relatedness between the GA2oxs. Crucial protein motifs were identified. Phylogenetic analysis revealed the evolutionary relationships between the GA2oxs. Three-dimensional structure of St-GA2ox1 was predicted by using AlphaFold tool, validated by the predicted local-distance difference test and Ramachandran Plot. Structural analysis and molecular docking were carried out to identify domains, binding sites and affinity for the ligand. The STRING database and hydropathy analysis revealed the presence of a putative interaction site for other enzymes. Expression Atlas database and semi-quantitative RT-PCR revealed the expression patterns of various GA2ox forms in different potato organs. This comprehensive report would be useful in providing new insights into possible underlying mechanisms involved in tuber development, and could facilitate the targeted alteration of genes responsible to combat the stress and enhance tuber production.









Similar content being viewed by others
References
Peters, R. J. (2013). Gibberellin phytohormone metabolism. In M. Rohmer & T. Bach (Eds.), Isoprenoid Synthesis in Plants and Microorganisms (pp. 233–249). New York: Springer.
Yamaguchi, S. (2008). Gibberellin metabolism and its regulation. Annual Review of Plant Biology, 59, 225–251.
Cheng, J., Ma, J., Zheng, X., Lv, H., Zhang, M., Tan, B., Ye, X., Wang, W., Zhang, L., Li, Z., Li, J., & Feng, J. (2021). Functional analysis of the Gibberellin 2-oxidase gene family in peach. Frontiers in Plant Science, 12, 619158.
Chen, S., Wang, X., Zhang, L., Lin, S., Liu, D., Wang, Q., Cai, S., El-Tanbouly, R., Gan, L., & Li, Y. (2016). Identification and characterization of tomato gibberellin 2-oxidases (GA2oxs) and effects of fruit-specific SlGA2ox1 overexpression on fruit and seed growth and development. Horticulture Research, 3, 16059.
Kawai, Y., Ono, E., & Mizutani, M. (2014). Evolution and diversity of the 2–oxoglutarate-dependent dioxygenase superfamily in plants. The Plant Journal, 78, 328–343.
Clifton, I. J., McDonough, M. A., Ehrismann, D., Kershaw, N. J., Granatino, N., & Schofield, C. J. (2006). Structural studies on 2-oxoglutarate oxygenases and related double-stranded beta-helix fold proteins. Journal of Inorganic Biochemistry, 100, 644–669.
Lange, T., & Pimenta Lange, M. J. (2020). The multifunctional dioxygenases of gibberellin synthesis. Plant and Cell Physiology, 61, 1869–1879.
Lo, S. F., Yang, S. Y., Chen, K. T., Hsing, Y. I., Zeevaart, J. A., Chen, L. J., & Yu, S. M. (2008). A novel class of gibberellin 2-oxidases control semidwarfism, tillering, and root development in rice. The Plant Cell, 20, 2603–2618.
Pearce, S., Huttly, A. K., Prosser, I. M., Li, Y. D., Vaughan, S. P., Gallova, B., Patil, A., Coghil, J. A., Dubcovsky, J., Hedden, P., & Phillips, A. L. (2015). Heterologous expression and transcript analysis of gibberellin biosynthetic genes of grasses reveals novel functionality in the GA3ox family. BMC Plant Biology, 15, 1–19.
Kim, G. B., Son, S. U., Yu, H. J., & Mun, J. H. (2019). MtGA2ox10 encoding C20-GA2-oxidase regulates rhizobial infection and nodule development in Medicago truncatula. Scientific Reports, 9, 1–13.
Ci, J., Wang, X., Wang, Q., Zhao, F., Yang, W., Cui, X., & Yang, W. (2021). Genome-wide analysis of gibberellin-dioxygenases gene family and their responses to GA applications in maize. PLoS ONE, 16, e0250349.
Yamauchi, Y., Takeda-Kamiya, N., Hanada, A., Ogawa, M., Kuwahara, A., Seo, M., Kamiya, Y., & Yamaguchi, S. (2007). Contribution of gibberellin deactivation by AtGA2ox2 to the suppression of germination of dark-imbibed Arabidopsis thaliana seeds. Plant Cell Physiology, 48, 555–561.
Li, C., Zheng, L., Wang, X., Hu, Z., Zheng, Y., Chen, Q., & Zhang, Y. (2019). Comprehensive expression analysis of Arabidopsis GA2-oxidase genes and their functional insights. Plant Science, 285, 1–13.
Shan, C., Mei, Z., Duan, J., Chen, H., Feng, H., & Cai, W. (2014). OsGA2ox5, a gibberellin metabolism enzyme, is involved in plant growth, the root gravity response and salt stress. PLoS ONE, 9, e87110.
Hu, Y. X., Tao, Y. B., & Xu, Z. F. (2017). Overexpression of Jatropha Gibberellin 2-oxidase 6 (JcGA2ox6) induces dwarfism and smaller leaves, flowers and fruits in Arabidopsis and Jatropha. Frontiers in Plant Science, 8, 2103.
Martínez-Bello, L., Moritz, T., & López-Díaz, I. (2015). Silencing C19-GA2-oxidases induces parthenocarpic development and inhibits lateral branching in tomato plants. Journal of Experimental Botany, 66, 5897–5910.
Sandoval-Oliveros, R., Guevara-Olvera, L., Beltrán, J. P., Gómez-Mena, C., & Acosta-García, G. (2017). Developmental landmarks during floral ontogeny of jalapeño chili pepper (Capsicum annuum L.) and the effect of gibberellin on ovary growth. Plant Reproduction, 30, 119–129.
Li, R., Sun, S., Wang, H., Wang, K., Yu, H., Zhou, Z., Xin, P., Chu, J., Zhao, T., Wang, H., & Cui, X. (2020). FIS1 encodes a GA2-oxidase that regulates fruit firmness in tomato. Nature Communications, 11, 1–12.
Dubois, M., Skirycz, A., Claeys, H., Maleux, K., Dhondt, S., De Bodt, S., Bossche, R. V., De Milde, L., Yoshizumi, T., Matsui, M., & Inzé, D. (2013). ETHYLENE RESPONSE FACTOR6 acts as a central regulator of leaf growth under water-limiting conditions in Arabidopsis. Plant Physiology, 162, 319–332.
Wang, Y., Cui, Y., Hu, G., Wang, X., Chen, H., Shi, Q., Xiang, J., Zhang, Y., Zhu, D., & Zhang, Y. (2018). Reduced bioactive gibberellin content in rice seeds under low temperature leads to decreased sugar consumption and low seed germination rates. Plant Physiology and Biochemistry, 133, 1–10.
Shi, J., Wang, J., Wang, N., Zhou, H., Xu, Q., & Yan, G. (2019). Overexpression of StGA2ox1 gene increases the tolerance to abiotic stress in transgenic potato (Solanum tuberosum L.) plants. Applied Biochemistry and Biotechnology, 187, 1204–1219.
Shi, J. B., Wang, N., Zhou, H., Xu, Q. H., & Yan, G. T. (2019). The role of gibberellin synthase gene GhGA2ox1 in upland cotton (Gossypium hirsutum L.) responses to drought and salt stress. Biotechnology and Applied Biochemistry, 66, 298–308.
Kloosterman, B., Vorst, O., Hall, R. D., Visser, R. G., & Bachem, C. W. (2005). Tuber on a chip: Differential gene expression during potato tuber development. Plant Biotechnology Journal, 3, 505–519.
Kloosterman, B., Navarro, C., Bijsterbosch, G., Lange, T., Prat, S., Visser, R. G. F., & Bachem, C. W. B. (2007). StGA2ox1 is induced prior to stolon swelling and controls GA levels during potato tuber development. The Plant Journal, 52, 362–373.
Roumeliotis, E., Visser, R. G., & Bachem, C. W. (2012). A crosstalk of auxin and GA during tuber development. Plant Signaling and Behavior, 7, 1360–1363.
Spooner, D. M., McLean, K., Ramsay, G., Waugh, R., & Bryan, G. J. (2005). A single domestication for potato based on multilocus amplified fragment length polymorphism genotyping. Proceedings of the National Academy of Sciences, 102, 14694–14699.
Dutt, S., Manjul, A. S., Raigond, P., Singh, B., Siddappa, S., Bhardwaj, V., & Kardile, H. B. (2017). Key players associated with tuberization in potato: Potential candidates for genetic engineering. Critical Reviews in Biotechnology, 37, 942–957.
Sambrook, J., Fritsch, E. F., & Maniatis, T. (1989). Molecular cloning: A laboratory manual. Cold Spring Harbor Laboratory Press.
Corpet, F. (1988). Multiple sequence alignment with hierarchical clustering. Nucleic Acids Research, 16, 10881–10890.
Jones, D. T. (1999). Protein secondary structure prediction based on position-specific scoring matrices. Journal of Molecular Biology, 292, 195–202.
Kumar, S., Stecher, G., Li, M., Knyaz, C., & Tamura, K. (2018). MEGA X: Molecular evolutionary genetics analysis across computing platforms. Molecular Biology and Evolution, 35, 1547–1549.
Felsenstein, J. (1985). Confidence limits on phylogenies: An approach using the bootstrap. Evolution, 39, 783–791.
Jumper, J., Evans, R., Pritzel, A., Green, T., Figurnov, M., Ronneberger, O., et al. (2021). Highly accurate protein structure prediction with alphafold. Nature, 596, 583–589.
Heo, L., Park, H., & Seok, C. (2013). Galaxyrefine: Protein structure refinement driven by side-chain repacking. Nucleic Acids Research, 41, W384–W388.
Laskowski, R. A., MacArthur, M. W., Moss, D. S., & Thornton, J. M. (1993). Procheck: A program to check the stereochemical quality of protein structures. Journal of Applied Crystallography, 26, 283–291.
Kessel, A., & Ben-Tal, N. (2002). Free energy determinants of peptide association with lipid bilayers. Current Topics in Membranes, 52, 205–253.
O’Boyle, N. M., Banck, M., James, C. A., Morley, C., Vandermeersch, T., & Hutchison, G. R. (2011). Open Babel: An open chemical toolbox. Journal of Cheminformatics, 3, 1–14.
Morris, G. M., Huey, R., Lindstrom, W., Sanner, M. F., Belew, R. K., Goodsell, D. S., et al. (2009). Autodock4 and autodocktools4: Automated docking with selective receptor flexibility. Journal of Computational Chemistry, 30, 2785–2791.
De Lano, W. L. (2002). Pymol: An open-source molecular graphics tool. CCP4 Newsletterson Protein Crystallography, 40, 82–92.
Gabler, F., Nam, S. Z., Till, S., Mirdita, M., Steinegger, M., Söding, J., et al. (2020). Protein sequence analysis using the Mpi bioinformatics toolkit. Current Protocols in Bioinformatics, 72, e108.
Milburn, D., Laskowski, R. A., & Thornton, J. M. (1998). Sequences annotated by structure: A tool to facilitate the use of structural information in sequence analysis. Protein Engineering, 11, 855–859.
Needleman, S. B., & Wunsch, C. D. A. (1970). General method applicable to the search for similarities in the amino acid sequence of two proteins. Journal of Molecular Biology, 48, 443–453.
Szklarczyk, D., Gable, A. L., Nastou, K. C., Lyon, D., Kirsch, R., Pyysalo, S., & von Mering, C. (2021). The STRING database in 2021: Customizable protein–protein networks, and functional characterization of user-uploaded gene/measurement sets. Nucleic Acids Research, 49, D605–D612.
Doolittle, R. (1989). Redundancies in protein sequences. In G. D. Fasman (Ed.), Prediction of protein structure and the principles of protein conformation (pp. 599–623). Plenum Press.
Enany, S. (2014). Structural and functional analysis of hypothetical and conserved proteins of Clostridium tetani. Journal of Infection and Public Health, 7, 296–307.
Saidi, A., & Hajibarat, Z. (2021). Genome wide identification of StKNOX gene family and characterization of their expression in Solanum tuberosum. Biocatalysis and Agricultural Biotechnology, 37, 102160.
Qin, F., Kodaira, K. S., Maruyama, K., Mizoi, J., Tran, L. S. P., Fujita, Y., & Yamaguchi-Shinozaki, K. (2011). SPINDLY, a negative regulator of gibberellic acid signaling, is involved in the plant abiotic stress response. Plant Physiology, 157, 1900–1913.
Sarkar, D. (2008). The signal transduction pathways controlling in planta tuberization in potato: An emerging synthesis. Plant Cell Reports, 27, 1–8.
Chen, H., Banerjee, A. K., & Hannapel, D. J. (2004). The tandem complex of BEL and KNOX partners is required for transcriptional repression of ga20ox1. The Plant Journal, 38, 276–284.
Marwaha, R. S., Pandey, S. K., Kumar, D., Singh, S. V., & Kumar, P. (2010). Potato processing scenario in India: Industrial constraints, future projections, challenges ahead and remedies−a review. Journal of Food Science and Technology, 47, 137–156.
Gill, S. C., & von Hippel, P. H. (1989). Calculation of protein extinction coefficients from amino acid sequence data. Analytical Biochemistry, 182, 319–326.
Gasteiger, E., Hoogland, C., Gattiker, A., Duvaud, S., Wilkins, M. R., Appel, R. D., & Bairoch, A. (2005). Protein identification and analysis tools on the ExPASy server. In J. M. Walker (Ed.), The proteomics protocols handbook (pp. 571–607). Humana.
Truebestein, L., & Leonard, T. A. (2016). Coiled-coils: The long and short of it. BioEssays, 38, 903–916.
Hu, L., Wang, P., Hao, Z., Lu, Y., Xue, G., Cao, Z., & Chen, J. (2021). Gibberellin oxidase gene family in L. chinense: Genome-wide identification and gene expression analysis. International Journal of Molecular Sciences, 22, 7167.
He, H., Liang, G., Lu, S., Wang, P., Liu, T., Ma, Z., & Mao, J. (2019). Genome-wide identification and expression analysis of GA2ox, GA3ox, and GA20ox are related to gibberellin oxidase genes in grape (Vitis Vinifera L.). Genes, 10, 680.
Zhang, C., Nie, X., Kong, W., Deng, X., Sun, T., Liu, X., & Li, Y. (2022). Genome-wide identification and evolution analysis of the gibberellin oxidase gene family in six gramineae crops. Genes, 13, 863.
Höfgen, R., & Willmitzer, L. (1990). Biochemical and genetic analysis of different patatin isoforms expressed in various organs of potato (Solanum tuberosum). Plant Science, 66, 221–230.
Gabaldón, T., & Koonin, E. V. (2013). Functional and evolutionary implications of gene orthology. Nature Reviews Genetics, 14, 360–366.
Kim, M. S., Kim, H. S., Kim, Y. S., Baek, K. H., Oh, H. W., Hahn, K. W., & Jeon, J. H. (2007). Superoxide anion regulates plant growth and tuber development of potato. Plant Cell Reports, 26, 1717–1725.
Thomas, S. G., Phillips, A. L., & Hedden, P. (1999). Molecular cloning and functional expression of gibberellin 2- oxidases, multifunctional enzymes involved in gibberellin deactivation. Proceedings of the National Academy of Sciences of the United States of America, 96, 4698–4703.
Song, L., Prince, S., Valliyodan, B., Joshi, T., Maldonado dos Santos, J. V., Wang, J., & Nguyen, H. T. (2016). Genome-wide transcriptome analysis of soybean primary root under varying water-deficit conditions. BMC Genomics, 17, 1–17.
Podell, S., & Gribskov, M. (2004). Predicting N-terminal myristoylation sites in plant proteins. BMC Genomics, 5, 1–15.
Li, Y., Shan, X., Jiang, Z., Zhao, L., & Jin, F. (2021). Genome-wide identification and expression analysis of the GA2ox gene family in maize (Zea mays L.) under various abiotic stress conditions. Plant Physiology and Biochemistry, 166, 621–633.
Acknowledgements
YK is thankful to Thapar Institute of Engineering & Technology (TIET), Patiala for providing financial support in the form of Teaching Associateship. The authors would like to acknowledge Department of Biotechnology, TIET, Patiala for providing necessary facilities to this work.
Author information
Authors and Affiliations
Contributions
Conceptualization, Methodology, Formal analysis and investigation, Writing-original draft preparation [Yadveer Kaur]; Writing-review and editing, Supervision [Niranjan Das].
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical Approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kaur, Y., Das, N. Gibberellin 2-Oxidases in Potato (Solanum tuberosum L.): Cloning, Characterization, In Silico Analysis and Molecular Docking. Mol Biotechnol 66, 902–917 (2024). https://doi.org/10.1007/s12033-023-00745-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12033-023-00745-8