Abstract
Cold shock proteins (CSPs) are small, acidic proteins which contain a conserved nucleic acid-binding domain. These perform mRNA translation acting as “RNA chaperones” when triggered by low temperatures initiating their cold shock response. CSP- RNA interactions have been predominantly studied. Our focus will be CSP-DNA interaction examination, to analyse the diverse interaction patterns such as electrostatic, hydrogen and hydrophobic bonding in both thermophilic and mesophilic bacteria. The differences in the molecular mechanism of these contrasting bacterial proteins are studied. Computational techniques such as modelling, energy refinement, simulation and docking were operated to obtain data for comparative analysis. The thermostability factors which stabilise a thermophilic bacterium and their effect on their molecular regulation is investigated. Conformational deviation, atomic residual fluctuations, binding affinity, Electrostatic energy and Solvent Accessibility energy were determined during stimulation along with their conformational study. The study revealed that mesophilic bacteria E. coli CSP have higher binding affinity to DNA than thermophilic G. stearothermophilus. This was further evident by low conformation deviation and atomic fluctuations during simulation.
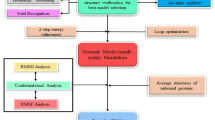
Graphical Abstract
















Similar content being viewed by others
Data Availability
All data generated and studies are available within this manuscriptand its supplementary article.
Code Availability
Not applicable.
Abbreviations
- CSP:
-
Cold shock proteins
- ssDNA:
-
Single-stranded DNA
- D1:
-
(DNA-binding site: residue symbol and position in the sequence)
- RMSD:
-
Root Mean Square Deviation
- RMSF:
-
Root Mean Square Fluctuation
- SASA:
-
Solvent-Accessible Surface Area
References
Graumann, P. L., & Marahiel, M. A. (1999). Cold Shock Response in Bacillus subtilis. Journal of molecular microbiology and biotechnology, 1, 203–209.
Zhou, Z., et al. (2021). A cold shock protein promotes high-temperature microbial growth through binding to diverse RNA species. Cell Discovery, 7, 15.
Metpally, R. P. R., & Reddy, B. V. B. (2009). Comparative proteome analysis of psychrophilic versus mesophilic bacterial species: Insights into the molecular basis of cold adaptation of proteins. BMC Genomics, 10, 11.
Schindler, T., Graumann, P. L., Perl, D., Ma, S., Schmid, F. X., & Marahiel, M. A. (1999). The family of cold shock proteins of Bacillus subtilis: Stability and dynamics in vitro and in vivo. Journal of Biological Chemistry, 274, 3407–3413.
Horn, G., Hofweber, R., Kremer, W., & Kalbitzer, H. R. (2007). Structure and function of bacterial cold shock proteins. Cellular and Molecular Life Sciences, 64, 1584.
Nakaminami, K., Karlson, D. T., & Imai, R. (2006). Functional conservation of cold shock domains in bacteria and higher plants. Biological Sciences, 103, 10122–10127.
SupachokSinchaikul, et al. (2002). Proteomic study of cold shock protein in Bacillus stearothermophilus P1: Comparison of temperature downshifts. Proteomics, 2, 1316–1324.
Fang, L. I., & Hou, Y. (1998). Role of the cold-box region in the 5 untranslated region of the cspA mRNA in its transient expression at low temperature in Escherichia coli. Journal of Bacteriology, 180, 90–95.
Oise Berger, F., Morellet, N., & F. Dé Ric Menu, and P. Potier,. (1996). Cold Shock and Cold Acclimation Proteins in the Psychrotrophic Bacterium Arthrobacter globiformis SI55. Journal of bacteriology, 178, 2999–3007.
Mueller, U., Perl, D., Schmid, F. X., & Heinemann, U. (2000). Thermal stability and atomic-resolution crystal structure of the Bacillus caldolyticus cold shock protein. Journal of Molecular Biology, 297, 975–988.
Schindelin, H., Jiangt, W., Inouyet, M., Heinemann, U., & Kristallographie, F. (1994). Crystal structure of CspA, the major cold shock protein of Escherichia coli. PNAS, 91, 5119–5123.
Sachs, R., Max, K. E. A., Heinemann, U., & Balbach, J. (2012). RNA single strands bind to a conserved surface of the major cold shock protein in crystals and solution. RNA, 18, 65–76.
Gasteigeretal, E. (1999). Protein analysis tools on the ExPASy server 571 571 From: The proteomics protocols handbook protein identification and analysis tools on the ExPASy server. Methods in Molecular Biology, 112, 531–552.
Chang, K. Y., & Yang, J. R. (2013). Analysis and prediction of highly effective antiviral peptides based on random forests. PLoS ONE, 8, e70166.
Aziz, M. F., & Caetano-Anollés, G. (2021). Evolution of networks of protein domain organization. Science and Reports, 11, 12075.
Porebski, B. T., & Buckle, A. M. (2016). Consensus protein design. Protein Engineering, Design and Selection, 29, 245–251.
Si, J., Zhao, R., & Wu, R. (2015). An overview of the prediction of protein DNA-binding sites. International Journal of Molecular Sciences, 16, 5194–5215.
Blum, M., et al. (2021). The InterPro protein families and domains database: 20 years on. Nucleic Acids Research, 49, D344–D354.
Waterhouse, A., et al. (2018). SWISS-MODEL: Homology modelling of protein structures and complexes. Nucleic Acids Research, 31, 3381–3385.
Ittisoponpisan, S., Islam, S. A., Khanna, T., Alhuzimi, E., David, A., & Sternberg, M. J. E. (2019). Can predicted protein 3d structures provide reliable insights into whether missense variants are disease associated ? Journal of molecular biology, 431, 2197–2212.
Wang, S., Li, W., Liu, S., & Xu, J. (2016). RaptorX-Property: A web server for protein structure property prediction. Nucleic Acids Research, 44, W430–W435.
Zheng, W., Zhang, C., Li, Y., Pearce, R., Bell, E. W., & Zhang, Y. (2021). Folding non-homologous proteins by coupling deep-learning contact maps with I-TASSER assembly simulations. Cell Reports Methods., 1, 100014.
Kim, D. E., Chivian, D., & Baker, D. (2004). Protein structure prediction and analysis using the Robetta server. Nucleic Acids Research, 32, W526–W531.
CHRIS COLOVOS and TODD O. YEATES,. (1993). Verification of protein structures: Patterns of nonbonded atomic interactions. Prorein Science, 2, 1511–1519.
Bowie, J. U., Ltcy, R., & Eisenberg, D. (2014). A method to identify protein sequences that fold into a known three-dimensional stucture. Science, 253, 164–170.
Laskowski, R. A., Antoon, J., Rullmann, C., MacArthur, M. W., Kaptein, R., & Thornton, J. M. (1996). AQUA and PROCHECK-NMR: Programs for checking the quality of protein structures solved by NMR*. Journal of Biomolecular NMR., 8, 477–486.
Wiederstein, M., & Sippl, M. J. (2007). ProSA-web: Interactive web service for the recognition of errors in three-dimensional structures of proteins. Nucleic Acids Research, 35, W407–W410.
Fiser, A., Do, R. K., & Šali, A. (2000). Modeling of loops in protein structures. Protein Science, 9(1753), 1773.
Xu, D., & Zhang, Y. (2011). Improving the physical realism and structural accuracy of protein models by a two-step atomic-level energy minimization. Biophysical Journal, 101, 2525–2534.
Pettersen, E. F., et al. (2004). UCSF Chimera - A visualization system for exploratory research and analysis. Journal of Computational Chemistry, 25, 1605–1612.
Krüger, D. M., Ahmed, A., & Gohlke, H. (2012). NMSim web server: Integrated approach for normal mode-based geometric simulations of biologically relevant conformational transitions in proteins. Nucleic Acids Research, 40, W310–W316.
Ahmed, A. (2011). A normal mode-based geometric simulation approach for exploring biologically relevant conformational transitions in proteins. Journal of Chemical Information and Modeling, 51, 1604–1622.
Martínez, L. (2015). Automatic identification of mobile and rigid substructures in molecular dynamics simulations and fractional structural fluctuation analysis. PLoS ONE, 10, e0119264.
Bornot, A., Etchebest, C., & de Brevern, A. G. (2011). “Predicting protein flexibility through the prediction of local structures”, Proteins: Structure. Function and Bioinformatics, 79, 839–852.
Heinig, M., & Frishman, D. (2004). STRIDE: A web server for secondary structure assignment from known atomic coordinates of proteins. Nucleic Acids Research, 32, W500–W502.
Klose, D. P., Wallace, B. A., & Janes, R. W. (2010). 2Struc: The secondary structure server. Bioinformatics, 26, 2624–2625.
Schindelin, H., et al. (1993). Universal nucleic acid-binding domain revealed by crystal structure of the B. subtilis major cold-shock protein. Nature, 364, 164–168.
Arnott, S., Campbell-Smith, P. J., & Chandrasekaran, R. (1976). Handbook of Biochemistry and Molecular Biology. Nucleic Acids., 2, 411–422.
Dominguez, C., Boelens, R., & Bonvin, A. M. J. J. (2003). HADDOCK: A protein-protein docking approach based on biochemical or biophysical information. Journal of the American Chemical Society, 125, 1731–1737.
Gabdoulline, R., Eckweiler, D., Kel, A., & Stegmaier, P. (2012). 3DTF: A web server for predicting transcription factor PWMs using 3D structure-based energy calculations. Nucleic Acids Research., 40, W180–W185.
Badaczewska-Dawid, A. E., Nithin, C., Wroblewski, K., Kurcinski, M., & Kmiecik, S. (2022). MAPIYA contact map server for identification and visualization of molecular interactions in proteins and biological complexes. Nucleic Acids Research, 50, W474–W482.
Fraczkiewicz, R., & Braun, W. (1998). Exact and efficient analytical calculation of the accessible surface areas and their gradients for macromolecules. Journal of Computational Chemistry, 19, 319–333.
Tokmakov, A. A., Kurotani, A., & Sato, K. I. (2021). Protein pI and Intracellular Localization. Frontiers in Molecular Biosciences, 8, 775736.
Enany, S. (2014). Structural and functional analysis of hypothetical and conserved proteins of Clostridium tetani. Journal of Infection and Public Health, 7, 296–307.
Aier, I., Varadwaj, P. K., & Raj, U. (2016). Structural insights into conformational stability of both wild-type and mutant EZH2 receptor. Scientific Reports, 6, 34984.
Bhattacharya, S., Dhar, S., Banerjee, A., & Ray, S. (2019). Structural, functional, and evolutionary analysis of late embryogenesis abundant proteins (LEA) in Triticum aestivum: a detailed molecular level biochemistry using in silico approach. Computational Biology and Chemistry, 82, 9–24.
Yu, B., Pettitt, B. M., & Iwahara, J. (2020). Dynamics of ionic interactions at protein-nucleic acid interfaces. Accounts of Chemical Research, 53, 1802–1810.
Tolstorukov, M. Y., Jernigan, R. L., & Zhurkin, V. B. (2004). Protein-DNA hydrophobic recognition in the minor groove is facilitated by sugar switching. Journal of Molecular Biology, 337, 65–76.
Ohlendorf, D. H., & Matthew, J. B. (1985). ELECTROSTATICS AND FLEXIBILITY IN PROTEIN-DNA INTERACTIONS. Advances in Biophysics, 20, 137–151.
Zhou, H. X., & Pang, X. (2018). Electrostatic interactions in protein structure, folding, binding, and condensation. Chemical Reviews, 118, 1691–1741.
Silverstein, T. P. (1998). The real reason why oil and water don’t mix. Journal of Chemical Education, 75, 116.
Harris, R. C., & Pettitt, B. M. (2016). Reconciling the understanding of “hydrophobicity” with physics-based models of proteins. Journal of Physics: Condensed Matter, 28, 083003.
Tanford, C. (1973). The hydrophobic effect: Formation of micelles and biological membranes (p. 58). Wiley.
Perrot, Pierre (1998), “A to Z of Thermodynamics.” Oxford University Press. ISBN 0–19–856552–6.
Halliday, David; Resnick, Robert (1988), “Fundamentals of Physics, Extended 3rd ed.” Wiley. ISBN 0–471–81995–6.
Wan, S., Bhati, A. P., Zasada, S. J., & P. v. Coveney,. (2020). Rapid, accurate, precise and reproducible ligand–protein binding free energy prediction. Interface Focus, 10, 20200007.
Mukherjee, S., & Bahadur, R. P. (2018). An account of solvent accessibility in protein-RNA recognition. Scientific Reports, 8, 10546.
Durham, E., Dorr, B., Woetzel, N., Staritzbichler, R., & Meiler, J. (2009). Solvent accessible surface area approximations for rapid and accurate protein structure prediction. Journal of Molecular Modeling, 15, 1093–1108.
Eswar, N., Ramakrishnan, C., & Srinivasan, N. (2003). Stranded in isolation: Structural role of isolated extended strands in proteins. Protein Engineering, 16, 331–339.
Receveur-Bréchot, V., & Durand, D. (2012). How random are intrinsically disordered proteins? A small angle scattering perspective. Current Protein and Peptide Science, 13, 55–75.
Acknowledgements
The authors would like to thank, Amity Institute of Biotechnology, Amity University, Kolkata, India, for their cooperation.
Funding
No funding support was received during this research work.
Author information
Authors and Affiliations
Contributions
AR and SR conceived of the presented idea. AR, did the calculation part. SR helped to select databases, softwares and webservers required for this study. Main backbone manuscript was written by AR. Tables, Figures were constructed by AR with the help of SR. Some portion of the result and discussion portion was specifically oriented by AR and SR. Overall Guidance and design were given by SR.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Roy, A., Ray, S. Traversing DNA-Protein Interactions Between Mesophilic and Thermophilic Bacteria: Implications from Their Cold Shock Response. Mol Biotechnol 66, 824–844 (2024). https://doi.org/10.1007/s12033-023-00711-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12033-023-00711-4