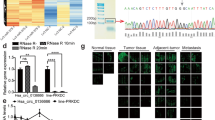
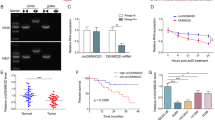
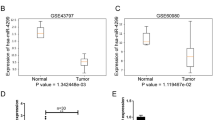
Abstract
The progression of gastric cancer (GC) is closely related to tumor immune escape. The research, therefore, studied the impact of possible circRNAs on the immune escape of GC tumors and the underlying mechanisms. Here, to explore circRNAs that may affect GC, the differential circRNAs in six normal gastric mucosal tissues and six GC samples (GSM2005868-GSM2005879) were analyzed through the bioinformatics website circmine, and hsa_circ_0076092 (circSCUBE3) was identified as the research object. In vitro assays revealed the functions of circSCUBE3 and its downstream miRNA/mRNA axis in GC cells. The effect of circSCUBE3 against PD-1 anti-tumor activity was evaluated in vivo. The relationship between circSCUBE3 and miR-744-5p, miR-744-5p, and SLC7A5 was identified by RNA immunoprecipitation and dual-luciferase reporter experiments. The effect of SLC7A5 on GC immune escape by regulating PD-L1 expression was assessed by co-culture system and flow cytometry. CircSCUBE3 was up-regulated in human GC tissues and GC cell lines. circSCUBE3 was associated with poor prognosis in GC patients. Functional experiments reported that circSCUBE3 knockdown could suppress GC immune escape. Mechanistically, circSCUBE3 bound to miR-744-5p, which further targeted SLC7A5, and SLC7A5 can affect GC immune escape by regulating PD-L1. Furthermore, in vivo assay manifested that circSCUBE3 attenuated the anti-tumor effect of PD-L1. Our study revealed the importance of the circSCUBE3/miR-744-5p/SLC7A5 axis in GC immune escape and anti-PD-1 resistance.









Similar content being viewed by others
References
Bray, F., et al. (2018). Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer Journal for Clinicians, 68(6), 394–424.
Sun, L., et al. (2021). H19 promotes aerobic glycolysis, proliferation, and immune escape of gastric cancer cells through the microRNA-519d-3p/lactate dehydrogenase A axis. Cancer Science, 112(6), 2245–2259.
Role of immunity in therapy of human cancer. Discussion. British Journal of Cancer Supplement, 1, 299–308.
Sikora, K., et al. (1991). Immune modulation and cancer. British Medical Bulletin, 47, 209–226.
Sadreddini, S., Baradaran, B., Aghebati-Maleki, A., et al. (2019). Immune checkpoint blockade opens a new way to cancer immunotherapy. Journal of Cell Physiology, 234, 8541–8549.
Rizvi, N., et al. (2015). Activity and safety of nivolumab, an anti-PD-1 immune checkpoint inhibitor, for patients with advanced, refractory squamous non-small-cell lung cancer (CheckMate 063): A phase 2, single-arm trial. The Lancet Oncology, 16(3), 257–265.
Brahmer, J. R., et al. (2012). Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. New England Journal of Medicine, 366, 2455–2465.
Chen, L., et al. (2015). Anti-PD-1/PD-L1 therapy of human cancer: Past, present, and future. Journal of Clinical Investigation, 125, 3384–3391.
Lin, Y., et al. (2022). A novel exosome-relevant molecular classification uncovers distinct immune escape mechanisms and genomic alterations in gastric cancer. Frontiers in Pharmacology, 13, 884090.
Chen, X., et al. (2021). ILT4 inhibition prevents TAM- and dysfunctional T cell-mediated immunosuppression and enhances the efficacy of anti-PD-L1 therapy in NSCLC with EGFR activation. Theranostics, 11, 3392–3416.
Tang, J., et al. (2022). Perioperative treatment and biomarker analysis of LP002, an anti-PD-L1 antibody, plus chemotherapy in resectable gastric and gastroesophageal junction cancer. Cancer Medicine. https://doi.org/10.1002/cam4.5414
Wang, F., et al. (2019). Safety, efficacy and tumor mutational burden as a biomarker of overall survival benefit in chemo-refractory gastric cancer treated with toripalimab, a PD-1 antibody in phase Ib/II clinical trial NCT02915432. Annals of Oncology: Official Journal of the European Society for Medical Oncology, 30(9), 1479–1486.
Tang, S., et al. (2020). Mechanisms of immune escape in the cancer immune cycle. International Immunopharmacology, 86, 106700.
Jiang, X., et al. (2019). Role of the tumor microenvironment in PD-L1/PD-1-mediated tumor immune escape. Molecular Cancer, 18(1), 10.
Wang, Z., et al. (2022). Membrane tension sensing molecule-FNBP1 is a prognostic biomarker related to immune infiltration in BRCA, LUAD and STAD. BMC Immunology, 23, 1.
Tian, Q., et al. (2022). Immunomodulatory functions of the circ_001678/miRNA-326/ZEB1 axis in non-small cell lung cancer via the regulation of PD-1/PD-L1 pathway. Human Molecular Genetics, 31(23), 4094–4106.
Xu, Y.-J., et al. (2021). Hsa_circ_0136666 activates Treg-mediated immune escape of colorectal cancer via miR-497/PD-L1 pathway. Cellular Signalling, 86, 110095.
Song, R., et al. (2020). Circular RNA MTO1 inhibits gastric cancer progression by elevating PAWR via sponging miR-199a-3p. Cell Cycle (Georgetown, Texas), 19(22), 3127–3139.
Shan, C., et al. (2019). Biogenesis, functions and clinical significance of circRNAs in gastric cancer. Molecular Cancer, 18(1), 136.
Necula, L., et al. (2019). Recent advances in gastric cancer early diagnosis. World Journal of Gastroenterology, 25(17), 2029–2044.
Lei, K., et al. (2018). The mechanism and function of circular RNAs in human diseases. Experimental Cell Research, 368(2), 147–158.
Lv, L., et al. (2022). Circular RNA hsa_circ_0026344 suppresses gastric cancer cell proliferation, migration and invasion via the miR-590-5p/PDCD4 axis. The Journal of Pharmacy and Pharmacology, 74(8), 1193–1204.
Li, B., et al. (2022). Circ_0008287 promotes immune escape of gastric cancer cells through impairing microRNA-548c-3p-dependent inhibition of CLIC1. International Immunopharmacology, 111, 108918.
Chen, D.-L., et al. (2021). The circular RNA circDLG1 promotes gastric cancer progression and anti-PD-1 resistance through the regulation of CXCL12 by sponging miR-141-3p. Molecular Cancer, 20, 166.
Yang, X., et al. (2020). Activation of TGF-β1 pathway by SCUBE3 regulates TWIST1 expression and promotes breast cancer progression. Cancer Biotherapy and Radiopharmaceuticals, 35(2), 120–128.
Xu, P., et al. (2022). SCUBE3 downregulation modulates hepatocellular carcinoma by inhibiting CCNE1 via TGFβ/PI3K/AKT/GSK3β pathway. Cancer Cell International, 22(1), 1.
Kurozumi, S., et al. (2022). Association of L-type amino acid transporter 1 (LAT1) with the immune system and prognosis in invasive breast cancer. Scientific Reports, 12, 2742.
Zhuang, Z., et al. (2022). Knockdown of circHIPK3 promotes the osteogenic differentiation of human bone marrow mesenchymal stem cells through activating the autophagy flux. FASEB Journal, 36, e22590.
Gao, Y., et al. (2022). Hsa_Circ_0066351 acts as a prognostic and immunotherapeutic biomarker in colorectal cancer. Frontiers in Immunology, 13, 927811.
Sun, Y., et al. (2022). CircSOX9 acts as a molecular sponge of miR-485-3p to promote the progression of nasopharyngeal carcinoma. Aging (Albany NY), 14, 4914–4926.
Hong, W., et al. (2020). Circular RNA circ-CPA4/let-7 miRNA/PD-L1 axis regulates cell growth, stemness, drug resistance and immune evasion in non-small cell lung cancer (NSCLC). Journal of Experimental and Clinical Cancer Research: CR, 39(1), 149.
Lin, J., et al. (2022). Hypoxia-induced exosomal circPDK1 promotes pancreatic cancer glycolysis via c-myc activation by modulating miR-628-3p/BPTF axis and degrading BIN1. Journal of Hematology and Oncology, 15, 128.
Chen, D., et al. (2021). The circular RNA circDLG1 promotes gastric cancer progression and anti-PD-1 resistance through the regulation of CXCL12 by sponging miR-141-3p. Molecular Cancer, 20(1), 166.
Shen, X., et al. (2018). Efficacy of PD-1 or PD-L1 inhibitors and PD-L1 expression status in cancer: Meta-analysis. BMJ (Clinical Research Edition), 362, k3529.
Zhou, R., et al. (2018). Circular RNAs (circRNAs) in cancer. Cancer Letters, 425, 134–142.
Chen, L. (2016). The biogenesis and emerging roles of circular RNAs. Nature Reviews Molecular Cell Biology, 17(4), 205–211.
Wei, J., et al. (2020). Circular RNA hsa_circRNA_102958 may serve as a diagnostic marker for gastric cancer. Cancer Biomarkers: Section A of Disease Markers, 27(2), 139–145.
Zhang, J., et al. (2021). A novel circular RNA circ_HN1/miR-628-5p/Ecto-5′-nucleotidase competing endogenous RNA network regulates gastric cancer development. Bioengineered, 12(2), 9739–9752.
Liu, P., et al. (2020). Circular RNA-hsa-circ-0000670 promotes gastric cancer progression through the microRNA-384/SIX4 axis. Experimental Cell Research, 394(2), 112141.
Song, J., et al. (2021). The circular RNA hsa_circ_000780 as a potential molecular diagnostic target for gastric cancer. BMC Medical Genomics, 14(1), 282.
Chang, C., et al. (2022). Circular RNA mitochondrial translation optimization 1 correlates with less lymph node metastasis, longer disease-free survival, and higher chemotherapy sensitivity in gastric cancer. Journal of Clinical Laboratory Analysis, 36(6), e23918.
Chen, S., et al. (2017). Using circular RNA hsa_circ_0000190 as a new biomarker in the diagnosis of gastric cancer. Clinica Chimica Acta International Journal of Clinical Chemistry, 466, 167–171.
Horlad, H., et al. (2016). An IL-27/Stat3 axis induces expression of programmed cell death 1 ligands (PD-L1/2) on infiltrating macrophages in lymphoma. Cancer Science, 107(11), 1696–1704.
Baudoux, T., et al. (2018). CD4 and CD8 T cells exert regulatory properties during experimental acute aristolochic acid nephropathy. Scientific Reports, 8(1), 5334.
Spranger, S. (2016). Mechanisms of tumor escape in the context of the T-cell-inflamed and the non-T-cell-inflamed tumor microenvironment. International Immunology, 28(8), 383–391.
Wu, A., et al. (2015). Reprogramming the tumor microenvironment: Tumor-induced immunosuppressive factors paralyze T cells. Oncoimmunology, 4(7), e1016700.
Wu, Z., et al. (2018). MiR-616-3p promotes angiogenesis and EMT in gastric cancer via the PTEN/AKT/mTOR pathway. Biochemical and Biophysical Research Communications, 501(4), 1068–1073.
Zhao, L., et al. (2020). miR-744-5p inhibits cellular proliferation and invasion via targeting ARF1 in epithelial ovarian cancer. The Kaohsiung Journal of Medical Sciences, 36(10), 799–807.
Chen, S., et al. (2019). miR-744-5p inhibits non-small cell lung cancer proliferation and invasion by directly targeting PAX2. Technology in Cancer Research and Treatment, 18, 1533033819876913.
Guo, T., et al. (2021). LncRNA PROX1-AS1 facilitates gastric cancer progression via miR-877-5p/PD-L1 axis. Cancer Management and Research, 13, 2669–2680.
Zhou, Z.-Q., et al. (2019). PD-L1 expression is a predictive biomarker for CIK cell-based immunotherapy in postoperative patients with breast cancer. Journal of Immunotherapy of Cancer, 7, 228.
Jancewicz, I., et al. (2021). PD-L1 overexpression, SWI/SNF complex deregulation, and profound transcriptomic changes characterize cancer-dependent exhaustion of persistently activated CD4 T cells. Cancers (Basel), 13(16), 4148.
Tumeh, P. C., et al. (2014). PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature, 515, 568–571.
Funding
Not applicable.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declared no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Shan, H., Zhang, X., Zhang, X. et al. CircSCUBE3 Reduces the Anti-gastric Cancer Activity of Anti-PD-L1. Mol Biotechnol 66, 123–137 (2024). https://doi.org/10.1007/s12033-023-00696-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12033-023-00696-0