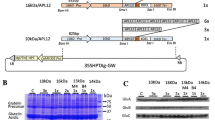
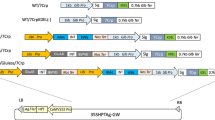
Abstract
To exploit the rice seed-based oral vaccine against Sjögren’s syndrome, altered peptide ligand of N-terminal 1 (N1-APL7) from its M3 muscarinic acetylcholine receptor (M3R) autoantigen was expressed as fusion protein with the representative four types of rice prolamins (16 kDa, 14 kDa, 13 kDa, and 10 kDa prolamins) under the control of the individual native prolamin promoter. The 10kD:N1-APL7 and 14kD:N1-APL7 accumulated at high levels (287 and 58 µg/grain), respectively, whereas production levels of the remaining ones were remarkably low. Co-expression of these fusion proteins did not enhance the accumulation level of N1-APL7 in an additive manner. Downregulation of endogenous seed storage proteins by RNAi-mediated suppression also did not lead to substantial elevation of the co-expressed prolamin:N1-APL7 products. When transgenic rice seeds were subjected to in vitro proteolysis with pepsin, the 10kD:N1-APL7 was digested more quickly than the endogenous 10 kDa prolamin and the 14kD:N1-APL7 deposited in PB-Is. This difference could be explained by the finding that the 10kD:N1-APL7 was unexpectedly localized in the PB-IIs containing glutelins. These results indicated that not only accumulation level but also subcellular localization of inherent prolamins were highly influenced by the liked N1-APL7 peptide.








Similar content being viewed by others
Abbreviations
- ALP:
-
Altered peptide ligand
- BiP:
-
Binding protein
- CBB:
-
Coomassie brilliant blue
- Cys:
-
Cysteine
- ER:
-
Endoplasmic reticulum
- M3R:
-
M3 muscarinic acetylcholine receptor
- PAC vesicle:
-
Precursor accumulating vesicle
- PB:
-
Protein body
- PDIL:
-
Protein disulfide isomerase-like
- PSV:
-
Protein-storage vacuole
- RNAi:
-
RNA interference
- SS:
-
Sjögren’s syndrome
- SSPs:
-
Seed storage proteins
References
Twyman, R. M., Stoger, E., Schillberg, S., Christou, P., & Fischer, R. (2003). Molecular farming in plants: Host systems and expression technology. Trends in Biotechnology, 21, 570–578.
Fischer, R., Stoger, E., Schillberg, S., Christou, P., & Twyman, R. M. (2004). Plant-based production of biopharmaceuticals. Current Opinion in Plant Biology, 7, 152–158.
Sharma, A. K., & Sharma, M. K. (2009). Plants as bioreactors: Recent developments and emerging opportunities. Biotechnology Advances, 27, 811–832.
Stoger, E., Ma, J. K., Fischer, R., & Christou, P. (2005). Sowing the seeds of success: Pharmaceutical proteins from plants. Current Opinion in Biotechnology, 16, 167–173.
Lau, O. S., & Sun, S. M. (2009). Plant seeds as bioreactors for recombinant protein production. Biotechnology Advances, 27, 1015–1022.
Takaiwa, F., Wakasa, Y., Hayashi, S., & Kawakatsu, T. (2017). An overview on the strategies to exploit rice endosperm as production platform for biopharmaceuticals. Plant Science, 263, 201–209.
Malonis, R. J., Lai, J. R., & Vergnolle, O. (2020). Peptide-based vaccines: Current progress and future challenges. Chemical Reviews, 120, 3210–3229.
Pihlanto, A., & Korhonen, H. (2003). Bioactive peptides and proteins. Advances in Food and Nutrition Research, 47, 175–276.
Udenigwe, C. C., & Aluko, R. E. (2012). Food protein-derived bioactive peptides: Production, processing, and potential health benefits. Journal of Food Science, 77, R11-24.
Maestri, E., Marmiroli, M., & Marmiroli, N. (2016). Bioactive peptides in plant-derived foodstuffs. Journal of Proteomics, 147, 140–155.
Duffuler, P., Bhullar, K. S., de Campos Zani, S. C., & Wu, J. (2022). Bioactive peptides: From basic research to clinical trials and commercialization. Journal of Agriculture and Food Chemistry, 70, 3585–3595.
Yang, L., Wakasa, Y., & Takaiwa, F. (2008). Biopharming to increase bioactive peptides in rice seed. Journal of AOAC International, 91, 957–964.
Wakasa, Y., & Takaiwa, F. (2013). The use of rice seeds to produce human pharmaceuticals for oral therapy. Biotechnology Journal, 8, 1133–1143.
Kawakatsu, T., Yamamoto, M. P., Hirose, S., Yano, M., & Takaiwa, F. (2008). Characterization of a new rice glutelin gene GluD-1 expressed in the starchy endosperm. Journal of Experimental Botany, 59, 4233–4245.
Takahashi, H., Saito, Y., Kitagawa, T., Morita, S., Masumura, T., & Tanaka, K. (2005). A novel vesicle derived directly from endoplasmic reticulum is involved in the transport of vacuolar storage proteins in rice endosperm. Plant and Cell Physiology, 46, 245–249.
Kawakatsu, T., & Takaiwa, F. (2010). Cereal seed storage protein synthesis: Fundamental processes for recombinant protein production in cereal grains. Plant Biotechnology Journal, 8, 939–953.
Wakasa, Y., Yang, L., Hirose, S., & Takaiwa, F. (2009). Expression of unprocessed glutelin precursor alters polymerization without affecting trafficking and accumulation. Journal of Experimental Botany, 60, 3503–3511.
Nagamine, A., Matsusaka, H., Ushijima, T., Kawagoe, Y., Ogawa, M., Okita, T. W., & Kumamaru, T. (2011). A role for the cysteine-rich 10 kDa prolamin in protein body I formation in rice. Plant and Cell Physiology, 52, 1003–1016.
Saito, Y., Shigemitsu, T., Yamasaki, R., Sasou, A., Goto, F., Kishida, K., Kuroda, M., Tanaka, K., Morita, S., Satoh, S., & Masumura, T. (2012). Formation mechanism of the internal structure of type I protein bodies in rice endosperm: Relationship between the localization of prolamin species and the expression of individual genes. The Plant Journal, 70, 1043–1055.
Sasou, A., Shigemitsu, T., Saito, Y., Tanaka, M., Morita, S., & Masumura, T. (2016). Control of foreign polypeptide localization in specific layers of protein body type I in rice seed. Plant Cell Reports, 35, 1287–1295.
Iizuka, M., Wakasa, Y., Tsuboi, H., Asashima, H., Hirota, T., Kondo, Y., Matsumoto, I., Takaiwa, F., & Sumida, T. (2014). Suppression of collagen-induced arthritis by oral administration of transgenic rice seeds expressing altered peptide ligands of type II collagen. Plant Biotechnology Journal, 12, 1143–1152.
Hirota, T., Tsuboi, H., Iizuka-Koga, M., Takahashi, H., Asashima, H., Yokosawa, M., Kondo, Y., Ohta, M., Wakasa, Y., Matsumoto, I., Takaiwa, F., & Sumida, T. (2017). Suppression of glucose-6-phosphate-isomerase induced arthritis by oral administration of transgenic rice seeds expressing altered peptide ligands of glucose-6-phosphate-isomerase. Modern Rheumatology, 27, 457–465.
Takagi, H., Hiroi, T., Yang, L., Tada, Y., Yuki, Y., Takamura, K., Ishimitsu, R., Kawauchi, H., Kiyono, H., & Takaiwa, F. (2005). A rice-based edible vaccine expressing multiple T cell epitopes induces oral tolerance for inhibition of Th2-mediated IgE responses. Proceedings of the National Academy of Sciences, 102, 17525–17530.
Yasuda, H., Hayashi, Y., Jomori, T., & Takaiwa, F. (2006). The correlation between expression and localization of a foreign gene product in rice endosperm. Plant and Cell Physiology, 47, 756–763.
Yang, L., Tada, Y., Yamamoto, M. P., Zhao, H., Yoshikawa, M., & Takaiwa, F. (2006). A transgenic rice seed accumulating an anti-hypertensive peptide reduces the blood pressure of spontaneously hypertensive rats. FEBS Letters, 580, 3315–3320.
Wakasa, Y., Yasuda, H., & Takaiwa, F. (2006). High accumulation of bioactive peptide in transgenic rice seeds by expression of introduced multiple genes. Plant Biotechnology Journal, 4, 499–510.
Gordon, B. P., & Guy, H. C. (2007). Regulation of salivary gland function by autonomic nerves. Autonomic Neuroscience, 133, 3–18.
Bacman, S., Berra, A., Sterin-Borda, L., & Borda, E. (2001). Muscarinic acetylcholine receptor antibodies as a new marker of dry eye Sjögren’s syndrome. Investigative Ophthalmology & Visual Science, 42, 321–327.
Naito, Y., Matsumoto, I., Wakamatsu, E., Goto, D., Sugiyama, T., Matsumura, R., Ito, S., Tsutsumi, A., & Sumida, T. (2005). Muscarinic acetylcholine receptor autoantibodies in patients with Sjögren’s syndrome. Annals of the Rheumatic Diseases, 64, 510–511.
Asashima, H., Tsuboi, H., Takahashi, H., Hirota, T., Iizuka, M., Kondo, Y., Matsui, M., Matsumoto, I., & Sumida, T. (2015). The anergy induction of M3 muscarinic acetylcholine receptor–reactive CD4+ T cells suppresses experimental sialadenitis-like Sjögren’s syndrome. Arthritis & Rhematology, 67, 2213–2225.
Sloan-Lancaster, J., & Allen, P. M. (1996). Altered peptide ligand-induced partial T cell activation: Molecular mechanisms and role in T cell biology. Annual Review of Immunology, 14, 1–27.
Kudo, H., Tsuboi, H., Asashima, H., Takahashi, H., Ono, Y., Abe, S., Honda, F., Kondo, Y., Wakasa, Y., Takaiwa, F., Takano, M., Matsui, M., Matsumoto, I., & Sumida, T. (2020). Transgenic rice seeds expressing altered peptide ligands against the M3 muscarinic acetylcholine receptor suppress experimental sialadenitis-like Sjögren’s syndrome. Modern Rheumatology, 30, 884–893.
Takagi, H., Hiroi, T., Hirose, S., Yang, L., & Takaiwa, F. (2010). Rice seed ER-derived protein body as an efficient delivery vehicle for oral tolerogenic peptides. Peptides, 31, 1421–1425.
Takaiwa, F., Wakasa, Y., Takagi, H., & Hiroi, T. (2015). Rice seed for delivery of vaccines to gut mucosal immune tissues. Plant Biotechnology Journal, 13, 1041–1055.
Shigemitsu, T., Ozaki, S., Saito, Y., Kuroda, M., Morita, S., Satoh, S., & Masumura, T. (2012). Production of human growth hormone in transgenic rice seeds: Co-introduction of RNA interference cassette for suppressing the gene expression of endogenous storage proteins. Plant Cell Reports, 31, 539–549.
Yang, L., Hirose, S., Takahashi, H., Kawakatsu, T., & Takaiwa, F. (2012). Recombinant protein yield in rice seed is enhanced by specific suppression of endogenous seed proteins at the same deposit site. Plant Biotechnology Journal, 10, 1035–1045.
Takaiwa, F. (2013). Increasing the production yield of recombinant protein in transgenic seeds by expanding the deposition space within the intracellular compartment. Bioengineered, 4, 136–139.
Kurokawa, S., Kuroda, M., Mejima, M., Nakamura, R., Takahashi, Y., Sagara, H., Takeyama, N., Satoh, S., Kiyono, H., Teshima, R., Masumura, T., & Yuki, Y. (2014). RNAi-mediated suppression of endogenous storage proteins leads to a change in localization of overexpressed cholera toxin B-subunit and the allergen protein RAG2 in rice seeds. Plant Cell Reports, 33, 75–87.
Takaiwa, F., Wakasa, Y., Ozawa, K., & Sekikawa, K. (2021). Improvement of production yield and extraction efficacy of recombinant protein by high endosperm-specific expression along with simultaneous suppression of major seed storage proteins. Plant Science, 302, 110692.
Takaiwa, F., Yang, L., Wakasa, Y., & Ozawa, K. (2018). Compensatory rebalancing of rice prolamins by production of recombinant prolamin/bioactive peptide fusion proteins within ER-derived protein bodies. Plant Cell Reports, 37, 209–223.
Kawakatsu, T., Hirose, Y., Yasuda, H., & Takaiwa, F. (2010). Reducing rice seed storage protein accumulation leads to changes in nutrient quality and storage organelle formation. Plant Physiology, 154, 1842–1854.
Goto, F., Yoshihara, T., Sugimoto, N., Toki, S., & Takaiwa, F. (1999). Iron fortification of rice seed by the soybean ferritin gene. Nature Biotechnology, 17, 282–286.
Tada, Y., Utsumi, S., & Takaiwa, F. (2003). Foreign gene products can be enhanced by introduction into storage protein mutants. Plant Biotechnology Journal, 1, 411–422.
Oono, Y., Wakasa, Y., Hirose, S., Yang, L., Sakuta, C., & Takaiwa, F. (2010). Analysis of ER stress in developing rice endosperm accumulating β-amyoid peptide. Plant Biotechnology Journal, 8, 691–718.
Wakasa, Y., Yasuda, H., Oono, Y., Kawakatsu, T., Hirose, S., Takahashi, H., Hayashi, S., Yang, L., & Takaiwa, F. (2011). Expression of ER quality control related genes in response to changes in BiP1 levels in developing rice endosperm. The Plant Journal, 65, 675–689.
Wakasa, Y., Hayashi, S., & Takaiwa, F. (2012). Expression of OsBiP4 and OsBiP5 is highly correlated with the endoplasmic reticulum stress response in rice. Planta, 236, 1519–1527.
Ohta, M., Wakasa, Y., Takahashi, H., Hayashi, S., Kudo, K., & Takaiwa, F. (2013). Analysis of rice ER-resident J-proteins reveals diversity and functional differentiation of the ER-resident Hsp70 system in plants. Journal of Experimental Botany, 64, 5429–5441.
Ohta, M., & Takaiwa, F. (2020). OsERdj7 is an ER-resident J-protein involved in ER quality control in rice endosperm. Journal of Plant Physiology, 245, 153109.
Yasuda, H., Hirose, S., Kawakatsu, T., Wakasa, Y., & Takiwa, F. (2009). Overexpression of BiP has inhibitory effects on the accumulation of seed storage proteins in endosperm cells of rice. Plant and Cell Physiology, 50, 1532–1543.
Yang, L., Kajiura, H., Suzuki, K., Hirose, S., Fujiyama, K., & Takaiwa, F. (2008). Generation of a transgenic rice seed-based edible vaccine against house dust mite allergy. Biochemical and Biophysical Research Communications, 365, 334–339.
Onda, Y., Nagamine, A., Sakurai, M., Kumamaru, T., Ogawa, M., & Kawagoe, Y. (2011). Distinct roles of protein disulfide isomerase and P5 sulfhydryl oxidoreductases in multiple pathways for oxidation of structurally diverse storage proteins in rice. The Plant Cell, 23, 210–223.
Wu, Y., & Messing, J. (2014). Proteome balancing of the maize seed for higher nutritional value. Frontiers in Plant Science, 5, 240.
Herman, E. M. (2014). Soybean seed proteome rebalancing. Frontiers in Plant Science, 5, 437.
Morandini, F., Avesani, L., Bortesi, L., Van Droogenbroeck, B., De Wilde, K., Arcalis, E., Bazzoni, F., Santi, L., Brozzetti, A., Falorni, A., Stoger, E., Depicker, A., & Pezzotti, M. (2011). Non-food/feed seeds as biofactories for the high-yield production of recombinant pharmaceuticals. Plant Biotechnology Journal, 9, 911–921.
Wang, S., Takahashi, H., Kajiura, H., Kawakatsu, T., Fujiyama, K., & Takaiwa, F. (2013). Transgenic rice seeds accumulating recombinant hypoallergenic birch pollen allergen Bet v 1 generate giant protein bodies. Plant and Cell Physiology, 54, 917–933.
Qu, L. Q., & Takaiwa, F. (2004). Evaluation of tissue specificity and expression strength of rice seed component gene promoters in transgenic rice. Plant Biotechnology Journal, 2, 113–125.
Pedrazzini, E., Mainieri, D., Marrano, C. A., & Vitale, A. (2016). Where do protein bodies of cereal seeds come from? Frontiers Plant Sci., 7, 1139.
Shewry, P. R., & Halford, N. G. (2002). Cereal seed storage proteins: Structures, properties and role in grain utilization. Journal of Experimental Botany, 370, 947–958.
Conrad, U., & Fiedler, U. (1998). Compartment-specific accumulation of recombinant immunoglobulins in plant cells: An essential tool for antibody production and immunomodulation of physiological functions and pathogen activity. Plant Molecular Biology, 38, 101–109.
Avesani, L., Falorni, A., Tornielli, G. B., Marusic, C., Porceddu, A., Polverari, A., Faleri, C., Calcinaro, F., & Pezzotti, M. (2003). Improved in planta expression of the human islet autoantigen glutamic acid decarboxylase (GAD65). Transgenic Research, 12, 203–212.
Takagi, H., Saito, S., Yang, L., Nagasaka, S., Nishizawa, N., & Takaiwa, F. (2005). Oral immunotherapy against a pollen allergy using a seed-based peptide vaccine. Plant Biotechnology Journal, 3, 521–533.
Acknowledgements
The author thanks Ms. Y. Ikemoto and Y. Yajima for technical assistance.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Takaiwa, F. Influence on Accumulation Levels and Subcellular Localization of Prolamins by Fusion with the Functional Peptide in Transgenic Rice Seeds. Mol Biotechnol 65, 1869–1886 (2023). https://doi.org/10.1007/s12033-023-00666-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12033-023-00666-6