Abstract
Main conclusion
A recombinant antigen targeting α-synuclein was produced in the plant cell rendering an immunogenic protein capable to induce humoral responses in mice upon oral administration.
Synucleinopathies are neurodegenerative diseases characterized by the abnormal accumulation of α-synuclein (α-Syn, a 140 amino acid protein that normally plays various neurophysiologic roles) aggregates. Parkinson’s disease (PD) is the synucleinopathy with the highest epidemiologic impact and although its etiology remains unknown, α-Syn aggregation during disease progression pointed out α-Syn as target in the development of immunotherapies. Herein a chimeric protein, comprising the B subunit of the enterotoxin from enterotoxigenic Escherichia coli and α-Syn epitopes, was expressed in the plant cell having the potential to induce humoral responses following oral immunization. This approach will serve as the basis for the development of oral plant-based vaccines against PD with several potential advantages such as low cost, easy scale-up during production, and easy administration.
Similar content being viewed by others
Introduction
α-Synucleinopathies are neurodegenerative diseases characterized by the abnormal accumulation of α-synuclein (α-Syn) aggregates in neurons, nerve fibres, or glial cells; comprising three pathologies: Parkinson’s disease (PD), dementia with Lewy bodies, and multiple system atrophy (Martí et al. 2003). In addition, about 50% of the studied cases of Alzheimer’s disease present α-Syn inclusions in structures with cognitive functions (McGeer and McGeer 2008).
Parkinson’s disease (PD) is the synucleinopathy with higher epidemiologic impact, in which α-Syn filaments (10–15 nm wide) are the main pathological sign at the cellular level (Goedert 2001). α-Syn is a small and soluble 140-amino acid protein that is predominantly expressed in the brain (Uversky 2003); specifically around the thalamus, striatum, olfactory bulb, hippocampus, neocortex, and cerebellum in the rat brain (Iwai et al. 1995).
In spite of the intensive research over the last three decades; the knowledge on the physiologic function of α-Syn is limited. It is known that the α-Syn overexpression produces adverse effects, which are not shown when its expression is abolished (Ibáñez et al. 2004; Burré et al. 2012). The presynaptic localization of α-Syn suggests a regulatory function associated with synaptic activity, synaptic plasticity, learning, neurotransmitter release, synaptic vesicle pool maintenance, and/or vesicle trafficking (Burré 2015).
The cause of PD remains unknown, but the following factors are associated to the pathogenesis: aging, environmental factors, oxidative stress, mitochondrial dysfunction, genetic factors, and dysfunction of the ubiquitin–proteasome system (Jellinger 2015). Another important factor in the PD pathology is neuroinflammation since chronic microglial activation, which causes a sustained IL-1, IL-6, TNF, ROS, and nitric oxide production in the brain, leads to a persistent detrimental damage to neurons that are coping with α-Syn aggregation (Sanchez-Guajardo et al. 2013a, b). Although current PD treatments are only palliative; the development of immunotherapies against non-communicable diseases, such as a diabetes and Alzheimer’s disease, has generated positive expectation and PD is a candidate for which vaccines could play a key role (Bachmann and Whitehead 2013).
Among the innovative platforms for vaccine production generated by the advances in Biotechnology, plants can be used as bio-factories capable of producing functional, cheap, and safe biopharmaceuticals. In addition, the plant cell can serve as the delivery vehicle of oral formulations. Several vaccine candidates produced in plants have generated positive outcomes at both preclinical and clinical evaluations (Yusibov et al. 2011). On the field of neurodegenerative diseases, several plant-based vaccine candidates against Alzheimer’s disease have been developed with some of them having a promising potential from results using transgenic mouse models (Rosales-Mendoza et al. 2014).
To develop an oral vaccine, a critical factor is the use of transmucosal carriers that favor the immunogenicity of the target antigen since antigen recognition occurs efficiently at the submucosa where dendritic cells and lymphocytes trigger adaptive immune responses. For this purpose, the enterotoxigenic E. coli heat labile enterotoxin B subunit (LTB) has been extensively used as an immunogenic carrier that exerts adjuvant effects to genetically fused unrelated epitopes (Lawson et al. 2011). This approach also leads to systemic humoral response that could exert therapeutic effects in diseases affecting tissues other than those belonging to the gastrointestinal tract (Salazar-Gonzalez et al. 2014; Kwon and Daniell 2016).
There is evidence indicating that the induction of humoral responses against α-Syn could serve as therapy for PD. Masliah et al. (2005) reported an approach based on the immunization with the full-length α-Syn, which reduced the number of α-Syn aggregates in a murine model of PD. Moreover, in this study the epitopes recognized by the induced antibodies were identified (Masliah et al. 2005). Epitopes α-Syn85–99, α-Syn109–126, and α-Syn126–140 were subsequently used as synthetic peptides conjugated to an adjuvant and tested in mice resulting in humoral responses able to recognize human α-Syn aggregates in brains from PD patients; suggesting a promising immunogenic activity for this formulation that could serve as vaccine (Ghochikyan et al. 2014). In addition, there is evidence that antibodies administered parenterally enters into the CNS (unknown mechanism) and binds to cells that display α-Syn accumulation leading to α-Syn clearance (Masliah et al. 2011; Valera and Masliah 2013).
In the present study, a plant-made candidate vaccine against PD was generated by expressing an LTB-based chimeric protein carrying α-Syn epitopes.
Materials and methods
Molecular cloning and plant transformation
A synthetic gene called LTB-Syn was designed to code the LTB-Syn chimeric protein, which comprises the signal peptide of a vegetative storage protein from Glycine max (MKMKVLVFFVATILVAWQCHA), the mature sequence of the enterotoxigenic E. coli heat labile toxin (LTB), a linker of six amino acids (DPRVPSR), an endoplasmic reticulum retention signal (SEKDEL), and three epitopes from α-Syn: α-Syn85–99 (AGSIAAATGFVKKDQ), α-Syn109–126 (QEGILEDMPVDPDNEAYE) and α-Syn126–140 (EMPSEEGYQDYEPEA). The codon-optimized gene was defined and synthesized by GenScript (Piscataway, NJ, USA).
A plant expression vector for the LTB-Syn gene was constructed by cloning into pBI121, which drives the expression by the Cauliflower Mosaic Virus 35S (CaMV35S) promoter and contains a kanamycin resistance gene (nptII) as selection marker. The cloning was performed using the restriction sites SmaI and SacI. The DNA fragments, LTB-Syn, and pBI121 lacking the uidA gene were purified from agarose gels and ligated to generate the pBI-LTB-Syn vector. A positive clone was identified and rescued by conventional procedures based on restriction profiles with SacI/HindIII and sequencing, which was subsequently transferred by electroporation to the GV3101 A. tumefaciens strain according to the method described by Cangelosi et al. (1991).
Tobacco (Nicotiana tabacum cv Petite Havana SR1) transformation was carried out by the method described by Horsch et al. (1985). Seeds were germinated on Murashige and Skoog (MS) medium until the development of plantlets with leaves of 1 cm in diameter, which were used as explants to perform the infection with an overnight-grown A. tumefaciens culture. The explants were subsequently blotted dry onto sterilized paper towels and co-cultivated for a period of 2 days using RMOP medium [MS salts and vitamins, supplemented with 0.1 mg/L naphthaleneacetic acid (NAA) and 1 mg/L 6-benzyladenine (BA)]. Thereafter the explants were placed on selective RMOP medium (RMOP supplemented with 100 mg/L kanamycin and 250 mg/L cefotaxime). All plates were kept in a controlled environment chamber (25 °C) under a 16 h-light/8 h-dark photoperiod. The explants were subcultured in fresh medium at 2-week intervals. The regenerated shoots of at least 1 cm in length rescued in selective medium were transferred to rooting medium (MS medium supplemented with 50 mg/L kanamycin and 250 mg/L cefotaxime). The rooted plantlets were acclimatized and transferred to a greenhouse.
Transgene detection
DNA was isolated from leaves of both transformed and wild-type plants according to Dellaporta et al. (1983). Two 25 µL PCR reaction mixtures were prepared to amplify transgenes, the first for the nptII gene (a 150 bp amplicon, sense 5′TGGCTGGAAAGAGGGAAATG, antisense 5′TGGGAATCAATGTGCTGACTAC) and the second for the LTB-Syn gene (a 91 bp amplicon, sense 5′TGGCTGGAAAGAGGGAAATG, antisense 5′TGGGAATCAATGTGCTGACTAC); which contained 1× PCR buffer, 100 ng DNA, 1.5 mM magnesium chloride, 2.5 U Taq DNA polymerase, 1 mM dNTPs, and 1 µM of primers. The cycling conditions were: 95 °C for 5 min (initial denaturation), 35 cycles at 95 °C for 30 s, 50 °C for 30 s, 72 °C for 30 s, and a final extension at 72 °C for 5 min. The PCR products were analyzed by electrophoresis in 1.5% agarose gels. The negative control consisted of 100 ng of DNA from a WT plant, whereas the positive control consisted of a reaction with 10 ng of the pBI-LTB-Syn vector.
Hyper-immune sera production
Anti-sera against α-Syn85–99, α-Syn109–126, α-Syn126–140, or LTB were obtained using 13-week-old BALB/c mice (n = 2). The test animals were immunized at day 1 into the rear foot pad with 10 μg of α-Syn synthetic peptides (GenScript) or LTB emulsified in 10 μL of complete Freund’s adjuvant (CFA). The subsequent doses were intraperitoneally administered on days 8, 15, and 22; consisting of 50 μg of the synthetic peptides or 25 μg of LTB emulsified in one volume of incomplete Freund’s adjuvant (IFA). Mice were bled at day 29 to measure antibody titers. The animals were subsequently killed to collect sera following research ethical committee standards for animal use.
LTB-Syn detection
The presence and integrity of the LTB-Syn chimeric protein was assessed by western-blot analysis. Protein extracts were obtained by grinding fresh leaf tissues and resuspending them in 500 µL of 1× reducing loading buffer [50 mM Tris–HCl (pH, 6.8), 100 mM DTT, 2% (w/v) SDS, 0.1% bromophenol blue, 10% (v/v) glycerol]. The samples were denatured by boiling them for 5 min at 95 °C eliminating debris by centrifugation at 13,300g for 10 min. The protein samples were subjected to SDS-PAGE in 4–12% acrylamide gels under denaturing conditions. LTB (500 ng) was used as positive control. The gel was subsequently blotted onto a BioTrace PVDF membrane. After blocking in PBST plus 5% fat-free milk; the membrane was labelled with the anti-LTB serum at a 1:400 dilution. Horseradish peroxidase-conjugated secondary antibody (1:2000 dilution) was added and incubation for 2 h at room temperature was performed. Immunodetection was revealed by incubation with SuperSignal West Dura solution following the instructions of the manufacturer. To detect positive signals, an X-ray film was exposed to the membrane that was subsequently treated with standard developer and fixer solutions.
The amount of protein in soluble extracts was estimated using ganglioside-dependent ELISA (GM1-ELISA) according to Chikwamba et al. (2002). Approximately 100 mg of fresh leaf tissues were minced in a mortar and homogenized in 200 µL of protein extraction buffer [25 mM sodium phosphate (pH 6.6), 100 mM NaCl, 0.5% Triton X-100 (v/v), and 1 mM PMSF] followed by centrifugation at 13,300g for 15 min at 4 °C. Three washes with PBST were applied to the ELISA plates between steps. The assay plates were coated with 1.5 µg of Type III GM1 ganglioside overnight at 4 °C, blocked with 5% fat free dry milk for 2 h at 25 °C, and incubated with plant extract overnight at 4 °C. The incubation with anti-LTB serum (1:400 dilution) was conducted at 25 °C for 4 h. The secondary antibody, a rabbit horseradish peroxidase-conjugated anti-mouse IgG, was added (1:2000 dilution) and plates were incubated for 1 h at 25 °C. An ABTS-based substrate solution [0.6 mM 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid), 0.1 M citric acid, pH 4.35 with 1 mM H2O2] was added. After 30 min of incubation at 25 °C, the OD at 405 nm was measured. A standard curve was constructed using pure LTB (donated by Dr. John Clements, Tulane University, USA) to estimate the expression levels in samples from transgenic plants.
Immunogenicity assessment
Immunogenicity of the LTB-Syn chimeric protein contained in tobacco biomass was assessed using 15-week-old female BALB/c mice of 25 g in body weight. The test animals were maintained under standard laboratory conditions with free access to food and water following the procedures indicated by the Federal Regulation for Animal Experimentation and Care (SAGARPA, NOM-062-ZOO-1999, México City, México). The protocol was approved by the Institutional Animal Care and Use Committee. The mice groups (n = 4) received, through a gastric cannula, one of the following treatments on days 1, 7, 14, and 21: 250 mg of fresh leaf tissues from transgenic line C3 homogenized in 500 µL of PBS; 250 mg of fresh leaf tissues from a WT plant homogenized in 500 µL of PBS. Mice were bled on days 14, 21, and 28. At day 21, serum samples were collected from blood extracted by drip from the lateral tail vein and stored at −70 °C until antibody content analysis.
ELISA assays were performed to determine the presence of specific antibodies in mice sera. The reactivity of antibodies was assessed against synthetic peptides carrying the target epitopes or a brain protein extract from rabbit obtained by homogenizing the tissue in phosphate buffer at a 1:3 volume ratio and a subsequent clarification by centrifugation at 13,300g for 15 min. ELISA assay plates were coated with antigens (1 µg of α-Syn85–99, α-Syn109–126, α-Syn126–140, or brain extract per well) overnight at 4 °C and subsequently blocked with 5% fat free dry milk for 2 h at 25 °C. The plates were further incubated overnight at 4 °C with serial dilutions of mice sera (1:10 to 1:40 dilutions). The secondary antibody, a mouse horseradish peroxidase-conjugated anti-mouse IgG, was added (1:2000 dilution) and the plates were incubated for 1 h at 25 °C. After washing, the substrate 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulphonic) (ABTS) with 1 mM H2O2 was added. After 60 min of incubation, the OD at 405 nm was measured. Samples were analyzed in triplicate and titers were calculated as the reciprocal of the last serum dilution yielding an OD value higher than the cut-off. The latter was calculated as the mean plus 2 SD of the OD values obtained with sera from mice immunized with WT tobacco.
Results
To achieve the expression of the LTB-Syn chimeric protein in plants the pBI121 binary vector was used to construct a functional plant expression vector for the LTB-Syn gene (Fig. 1). According to the restriction profile obtained by SacI and HindIII digestion, as well as conventional sequencing data (data not shown), the gene was successfully subcloned resulting in the pBI-LTB-Syn vector; in which the LTB-Syn expression is driven by the constitutive promoter CaMV35S and the nopaline synthase terminator (Fig. 2). Since the nptII marker gene is placed in the T-DNA region, the transformed lines can be rescued by kanamycin-based selection.
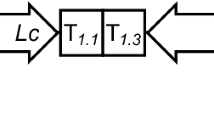
Amino acid sequence of the LTB-Syn protein. The immunogen design is based on the following elements: the signal peptide of the G. max vegetative storage protein (underlined letters), the mature sequence of the B subunit of the E. coli heat labile enterotoxin (segmented underlined letters), a flexible linker containing proline (bold letters), α-Syn epitopes (α-Syn85–99, α-Syn109–126, α-Syn126–140; gray letters), and an endoplasmic reticulum retention signal (box)
Description of the pBI-LTB-Syn expression vector. This construction is based on the binary vector pBI121 and was used to transform tobacco plants by A. tumefaciens. RB right border, LB left border. The vector harbors the nptII resistance gene under control of the nopaline synthase promoter for selection of transformants. The gene of interest is expressed under control of the cauliflower mosaic virus 35S constitutive promoter (CaMV35S)
The pBI-LTB-Syn plasmid was successfully mobilized into agrobacteria cells by electroporation and used for tobacco transformation. After 2 months under selective pressure, 12 putative transformed calli with active growth were rescued from transformed tobacco leaf tissues. Seven lines were randomly selected to complete regeneration, which took 3 months from co-culture. The phenotype of putative transgenic plants showed no differences to those plants regenerated from WT explants cultivated in absence of the selection agent. Thereafter, the putative transgenic plants were analyzed to assess the presence of the nptII and LTB-Syn genes. All the candidate lines were positive for this analysis, whereas a DNA sample from a WT plant showed no amplification (Fig. 3a, b).
Transgene detection in candidate plant lines. DNA samples from putative transformants and the WT line were analyzed by two different PCR using specific primers targeting either nptII or LTB-Syn genes. a Agarose gel analysis for the amplification of the nptII gene, where the presence of 150 bp amplicons indicates a specific amplification. Lanes: MWM molecular weight marker, (−) negative control reagent (water), (+) positive control (10 ng of pBI-LTB-Syn), B, C3, J2, K5, I9, E7, and H candidate transgenic lines, WT wild-type tobacco line. b Agarose gel analysis for the amplification of the LTB-Syn gene, where 91 bp amplicons indicate a specific amplification. Lanes: MWM molecular weight marker, (−) negative control reagent (water), (+) positive control (10 ng of pBI-LTB-Syn), B, C3, J2, K5, I9, E7, and H candidate transgenic lines, WT wild-type tobacco line
Hyperimmune sera were raised in mice to performed immunodetection assays targeting the antigenic determinants present in the chimeric protein. Only hyperimmune sera targeting α-Syn126–140 and LTB were successfully obtained in mice as revealed by ELISA assays targeting the specific antigens (data not shown), whereas the animals immunized with the peptides α-Syn85–99 and α-Syn109–126 showed no significant OD increase with respect to the negative control. Anti-α-Syn126–140 and -LTB sera were used to screen the transformed tobacco lines for the presence of the immunoreactive LTB-Syn protein through western-blot and ELISA analyses. First, a GM1-dependent ELISA was conducted using an anti-LTB hyperimmune serum to investigate the expression capacity as well as the proper folding of LTB-Syn. A standard curve was obtained with proper correlation values (Fig. 4a). Significant higher OD readings were recorded for the B and C3 lines when compared to the WT line (Fig. 4b). According to the standard curve data; the expression levels in these lines were 0.16 and 0.27 µg/g fresh tissue, respectively (Fig. 4c). When the α-Syn126–140 anti-serum was used for labelling; the ELISA assays revealed higher OD readings for the transgenic line C3 than those observed for the WT extract (Fig. 4d). To confirm the expression of the chimeric protein in tobacco a western-blot analysis was performed. Following labeling with specific anti-LTB serum, an 11 kDa band was detected for the positive control. In the case of the plant extracts, 21 kDa bands were detected for most of the transgenic lines (Fig. 5).
Detection of LTB-Syn by ELISA. Total soluble protein extracts from transgenic or WT plants were analyzed by a GM1-ELISA assay using anti-LTB or -α-Syn126–140 sera. a Standard curve (R = 0.9907) used for LTB quantification. b Optical density readings at 405 nm observed for transgenic and WT lines when an anti-LTB was used for labelling. c Accumulation levels of LTB-Syn in leaf tissues from transgenic lines expressed as µg of LTB-Syn per gram of fresh leaf tissue. d Optical density values at 405 nm observed for transgenic and WT lines when an anti-α-Syn126–140 serum was used for labelling
Following the immunization scheme the immunogenic activity of the plant-made LTB-Syn was estimated by ELISA assays targeting α-Syn85–99, α-Syn109–126, α-Syn126–140 or brain protein extracts from rabbit. Sera from mice immunized with the plant-made antigen showed significantly higher OD readings in ELISA assays targeting either the synthetic peptides α-Syn85–99, α-Syn109–126, α-Syn126–140 (Fig. 6a–c) or brain protein extract (Fig. 6d) when compared to the group treated with a WT plant. The average titers of anti-Syn IgG antibodies in serum were 40. This finding indicates the presence of anti-Syn IgG antibodies in sera from animals orally immunized with the LTB-Syn bioencapsulated in tobacco leaf tissue.
Discussion
In the present study, a chimeric protein was designed to target α-Syn in oral immunotherapies. The plant cell was used to express this candidate since it constitutes an attractive low cost biofactory for functional antigens and at the time can serve as an effective delivery vehicle. Transformed plants expressing the corresponding transgene under the control of the 35SCaMV promoter were obtained and successfully transferred to soil. The GM1-ELISA analysis showed positive reactivity of protein extracts from transgenic plants with an anti-LTB serum and a hyperimmune serum targeting α-Syn126–140, indicating that the chimeric protein is expressed in the plant cell retaining integrity and the antigenic determinants of the carrier protein; but also retaining the native oligomeric conformation since this assay is based on the specific binding of pentameric LTB to GM1.
Thus, this analysis suggests that the LTB-Syn protein is a functional antigen and supports further immunogenicity evaluations in test animals. The yields of plant leaf tissues were up to 0.15 µg/g fresh weight. Although the transgene in various lines was observed, the reason for the absence of protein expression could be associated to the insertion of the T-DNA in a silenced locus. The plant lines expressing the LTB-Syn protein did not exhibit phenotypic alterations (Fig. 7).
Based on the western-blot results the 21 kDa immunoreactive protein is likely to correspond to the monomeric form of the chimeric protein, which correlates with the theoretical molecular weight of 20.86 kDa predicted by a bioinformatic tool (http://www.expasy.org/).
LTB has been used in the design of several oral vaccine prototypes made in plants. The candidates were predominantly developed against infectious diseases, including candidates against the human papillomavirus (Waheed et al. 2011; Hongli et al. 2013) and the enterotoxigenic E. coli (Ravin et al. 2008; Soh et al. 2015). The capacity of the plant cell to produce LTB at proper levels retaining the immunogenicity when orally administered is well documented (Zhang et al. 2009; Miller et al. 2012; Pelosi et al. 2012). To date there are few studies focusing on creating plant-based vaccines with LTB for non-infectious conditions. For instance, Pinkhasov et al. (2011) produced a human tumor-associated antigen fused to LTB in Nicotiana benthamiana. Another study consisted in the expression of an immunocontraceptive epitope in tomato, genetically fused to LTB, at levels of 37.8 µg/g dry weight of transgenic tissues (Walmsley et al. 2003). The observed yields for our LTB-Syn (0.15 µg/g fresh weight) are lower to the yields reported for other plant-based vaccines. For instance, in tobacco the expression of CTB chimeras reached antigen accumulation up to 10 µg/g fresh weight in nuclear-transformed plants (Salazar-Gonzalez et al. 2014). However, the observed accumulation levels for LTB-Syn were sufficient to elicit immune responses in mice avoiding the possibility of phenotypic alterations; which were observed in potato plants expressing LTB at levels up to 17.2 µg/g fresh weight (Haq et al. 1995). The significantly higher OD readings observed in ELISA analysis of sera from orally immunized mice, with LTB-Syn, evidenced the induction of anti-Syn humoral responses targeting brain proteins; suggesting the presence of antibodies against α-Syn. Moreover, ELISA assays targeting the individual peptides confirmed the immunogenicity of each of the vaccine components, which accounts for the potential of the plant-based vaccine.
To the best of our knowledge, this is the first report on the expression of an antigen that could lead to plant-made vaccine candidates against PD. We used the epitopes previously described by Masliah et al. (2005) while other investigations have been based on the use of the full-length α-Syn (Reynolds et al. 2009; Sanchez-Guajardo et al. 2013a, b). The use of other α-Syn epitopes has been recently reported, generating parallel pathways for vaccine development (Ugen et al. 2015). The immediate perspectives for this study comprise the expression assessment of LTB-Syn in plant species yielding edible tissues, e.g., carrot or lettuce, as well as assessing the therapeutic potential in preclinical models.
In conclusion, the protein LTB-Syn can be expressed at convenient levels in the plant cell without side effects in plant development, retaining the antigenic determinants of LTB; which suggests a remarkable potential of this approach for designing plant-made vaccines against PD.
Author contribution statement
JIAV performed most of experiments. JIAV and SRM carried out the experimental design, data analysis and wrote the manuscript. EME and DOGA participated in test mice experiments. SZG contributed to the data analysis. All authors discussed the results, read and approved the final version of the manuscript.
Abbreviations
- α-Syn:
-
α-Synuclein
- LTB:
-
E. coli heat labile toxin B subunit
- PD:
-
Parkinson’s disease
References
Bachmann MF, Whitehead P (2013) Active immunotherapy for chronic diseases. Vaccine 31:1777–1784
Burré J (2015) The synaptic function of α-Synuclein. J Parkinsons Dis 5:699–713
Burré J, Sharma M, Südhof TC (2012) Systematic mutagenesis of α-synuclein reveals distinct sequence requirements for physiological and pathological activities. J Neurosci 32:15227–15242
Cangelosi GA, Best EA, Martinetti G, Nester EW (1991) Genetic analysis of Agrobacterium. Methods Enzymol 204:384–397
Chikwamba R, Cunnick J, Hathaway D, McMurray J, Mason H, Wang K (2002) A functional antigen in a practical crop: LT-B producing maize protects mice against Escherichia coli heat labile enterotoxin (LT) and cholera toxin (CT). Transgenic Res 11:479–493
Dellaporta SL, Wood J, Hicks JB (1983) A plant DNA minipreparation: version II. Plant Mol Biol Rep 1:19–21
Ghochikyan A, Petrushina I, Davtyan H, Hovakimyan A, Saing T, Davtyan A, Cribbs DH, Agadjanyan MG (2014) Immunogenicity of epitope vaccines targeting different B cell antigenic determinants of human α-synuclein: feasibility study. Neurosci Lett 560:86–91
Goedert M (2001) Alpha-synuclein and neurodegenerative diseases. Nat Rev Neurosci 2:492–501
Haq TA, Mason HS, Clements JD, Arntzen CJ (1995) Oral immunization with a recombinant bacterial antigen produced in transgenic plants. Science 268(5211):714–716
Hongli L, Xukui L, Ting L, Wensheng L, Lusheng S, Jin Z (2013) Transgenic tobacco expressed HPV16-L1 and LT-B combined immunization induces strong mucosal and systemic immune responses in mice. Hum Vaccines Immunother 9:83–89
Horsch RB, Fry JE, Hoffmann NL, Eichholtz D, Rogers SG, Fraley RT (1985) A simple and general method for transferring genes into plants. Science 227:1229–1231
Ibáñez P, Bonnet AM, Débarges B, Lohmann E, Tison F, Pollak P, Agid Y, Dürr A, Brice A (2004) Causal relation between alpha-synuclein gene duplication and familial Parkinson’s disease. Lancet 364:1169–1171
Iwai A, Masliah E, Yoshimoto M, Ge N, Flanagan L, de Silva HA, Kittel A, Saitoh T (1995) The precursor protein of non-A beta component of Alzheimer’s disease amyloid is a presynaptic protein of the central nervous system. Neuron 14:467–475
Jellinger KA (2015) How close are we to revealing the etiology of Parkinson’s disease? Expert Rev Neurother 15:1105–1107
Kwon KC, Daniell H (2016) Oral delivery of protein drugs bioencapsulated in plant cells. Mol Ther 24:1342–1350
Lawson LB, Norton EB, Clements JD (2011) Defending the mucosa: adjuvant and carrier formulations for mucosal immunity. Curr Opin Immunol 23:414–420
Martí MJ, Tolosa E, Campdelacreu J (2003) Clinical overview of the synucleinopathies. Mov Disord 18(Suppl 6):S21–S27
Masliah E, Rockenstein E, Adame A, Alford M, Crews L, Hashimoto M, Seubert P, Lee M, Goldstein J, Chilcote T, Games D, Schenk D (2005) Effects of alpha-synuclein immunization in a mouse model of Parkinson’s disease. Neuron 46:857–868
Masliah E, Rockenstein E, Mante M, Crews L, Spencer B, Adame A, Patrick C, Trejo M, Ubhi K, Rohn TT, Mueller-Steiner S, Seubert P, Barbour R, McConlogue L, Buttini M, Games D, Schenk D (2011) Passive immunization reduces behavioral and neuropathological deficits in an alpha-synuclein transgenic model of Lewy body disease. PLoS One 6(4):e19338
McGeer PL, McGeer EG (2008) The alpha-synuclein burden hypothesis of Parkinson disease and its relationship to Alzheimer disease. Exp Neurol 212:235–238
Miller T, Fanton M, Nickelson S, Mason H, Webb S (2012) Safety and immunogenicity of bacterial and tobacco plant cell line derived recombinant native and mutant Escherichia coli heat-labile toxin in chickens. Avian Pathol 41:441–449
Pelosi A, Piedrafita D, De Guzman G, Shepherd R, Hamill JD, Meeusen E, Walmsley AM (2012) The effect of plant tissue and vaccine formulation on the oral immunogenicity of a model plant-made antigen in sheep. PLoS One 7(12):e52907
Pinkhasov J, Alvarez ML, Rigano MM, Piensook K, Larios D, Pabst M, Grass J, Mukherjee P, Gendler SJ, Walmsley AM, Mason HS (2011) Recombinant plant-expressed tumour-associated MUC1 peptide is immunogenic and capable of breaking tolerance in MUC1.Tg mice. Plant Biotechnol J 9:991–1001
Ravin NV, Kuprianov VV, Zamchuk LA, Kochetov AV, Dorokhov YL, Atabekov JG, Skryabin KG (2008) Highly efficient expression of Escherichia coli heat-labile enterotoxin B subunit in plants using potato virus X-based vector. Biochemistry (Mosc) 73:1108–1113
Reynolds AD, Stone DK, Mosley RL, Gendelman HE (2009) Nitrated {alpha}-synuclein-induced alterations in microglial immunity are regulated by CD4+ T cell subsets. J Immunol 182:4137–4149
Rosales-Mendoza S, Rubio-Infante N, Zarazúa S, Govea-Alonso DO, Martel-Gallegos G, Moreno-Fierros L (2014) Plant-based vaccines for Alzheimer’s disease: an overview. Expert Rev Vaccines 13:429–441
Salazar-Gonzalez JA, Rosales-Mendoza S, Romero-Maldonado A, Monreal-Escalante E, Uresti-Rivera EE, Bañuelos-Hernández B (2014) Production of a plant-derived immunogenic protein targeting ApoB100 and CETP: toward a plant-based atherosclerosis vaccine. Mol Biotechnol 56:1133–1142
Sanchez-Guajardo V, Annibali A, Jensen PH, Romero-Ramos M (2013a) α-Synuclein vaccination prevents the accumulation of parkinson disease-like pathologic inclusions in striatum in association with regulatory T cell recruitment in a rat model. J Neuropathol Exp Neurol 72:624–645
Sanchez-Guajardo V, Barnum CJ, Tansey MG, Romero-Ramos M (2013b) Neuroimmunological processes in Parkinson’s disease and their relation to α-synuclein: microglia as the referee between neuronal processes and peripheral immunity. ASN Neuro 5:113–139
Soh HS, Chung HY, Lee HH, Ajjappala H, Jang K, Park JH, Sim JS, Lee GY, Lee HJ, Han YH, Lim JW, Choi I, Chung IS, Hahn BS (2015) Expression and functional validation of heat-labile enterotoxin B (LTB) and cholera toxin B (CTB) subunits in transgenic rice (Oryza sativa). Springerplus 4:148
Ugen KE, Lin X, Bai G, Liang Z, Cai J, Li K, Song S, Cao C, Sanchez-Ramos J (2015) Evaluation of an α synuclein sensitized dendritic cell based vaccine in a transgenic mouse model of Parkinson disease. Hum Vaccines Immunother 11:922–930
Uversky VN (2003) A protein-chameleon: conformational plasticity of alpha-synuclein, a disordered protein involved in neurodegenerative disorders. J Biomol Struct Dyn 21:211–234
Valera E, Masliah E (2013) Immunotherapy for neurodegenerative diseases: focus on α-synucleinopathies. Pharmacol Ther 138(3):311–322
Waheed MT, Thönes N, Müller M, Hassan SW, Gottschamel J, Lössl E, Kaul HP, Lössl AG (2011) Plastid expression of a double-pentameric vaccine candidate containing human papillomavirus-16 L1 antigen fused with LTB as adjuvant: transplastomic plants show pleiotropic phenotypes. Plant Biotechnol J 9:651–660
Walmsley AM, Alvarez ML, Jin Y, Kirk DD, Lee SM, Pinkhasov J, Rigano MM, Arntzen CJ, Mason HS (2003) Expression of the B subunit of Escherichia coli heat-labile enterotoxin as a fusion protein in transgenic tomato. Plant Cell Rep 21(10):1020–1026
Yusibov V, Streatfield SJ, Kushnir N (2011) Clinical development of plant-produced recombinant pharmaceuticals: vaccines, antibodies and beyond. Hum Vaccines 7:313–321
Zhang X, Yuan Z, Duan Q, Zhu H, Yu H, Wang Q (2009) Mucosal immunity in mice induced by orally administered transgenic rice. Vaccine 27:1596–1600
Acknowledgements
We acknowledge Andrea Romero-Maldonado for helping on anti-sera production and Dania O. Govea-Alonso for providing plant material. The current investigations from the group are supported by CONACYT/México (Grant INFR-2016-271182 and CB-256063 to SRM).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interests.
Rights and permissions
About this article
Cite this article
Arevalo-Villalobos, J.I., Govea-Alonso, D.O., Monreal-Escalante, E. et al. LTB-Syn: a recombinant immunogen for the development of plant-made vaccines against synucleinopathies. Planta 245, 1231–1239 (2017). https://doi.org/10.1007/s00425-017-2675-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00425-017-2675-y