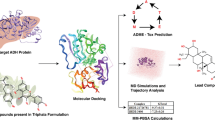
Abstract
Diabetes is a serious growing concern that affects many parts of the body including the skin due to high sugar levels. Moreover, diabetic patients are at risk of developing cancer and are prone to a higher risk of hematological malignancies. In the present study, the inhibitory effect of 2′-Hydroxy-4′,5′-dimethoxyacetophenone was investigated on aldose reductase and collagenase enzymes, along with docking and ADMET analysis. MTT assay was also conducted to investigate the anti-leukemic effect of 2′-Hydroxy-4′,5′-dimethoxyacetophenone on human acute leukemia cells (32D-FLT3-ITD, Human HL-60/vcr, MOLT-3, and TALL-104 cell lines) and DPPH assay for establishing activity against oxidative stress. The 2′-Hydroxy-4′,5′-dimethoxyacetophenone showed potent inhibition of both the above tested enzymes with numerous strong interactions with the key catalytic residues in the active site of the enzymes. The MTT assay showed strong anti-cancer activity against entire tested human acute leukemia cells and was found non-toxic to normal (HUVEC) at the tested concentration. In DPPH free radical scavenging assay, 2′-Hydroxy-4′,5′-dimethoxyacetophenone showed strong inhibitory activity with IC50 of 157 µg/mL, which found comparable to the standard BHT. Our study demonstrated prominent pharmacological benefit of 2′-Hydroxy-4′,5′-dimethoxyacetophenone, against various leukemic cell lines, aldose reductase and collagenase enzymes, and free radical scavenging activity.





Similar content being viewed by others
Abbreviations
- ADMET:
-
Absorption, distribution, metabolism, excretion, and toxicity
- MTT:
-
(3-[4,5-Dimethylthiazol-2-yl]-2,5 diphenyl tetrazolium bromide)
- HUVECs:
-
Human umbilical vein endothelial cells
- DPPH:
-
Diphenylpicrylhydrazyl
- BHT:
-
Butylated hydroxytoluene
- ROS:
-
Reactive oxygen species
- NADPH:
-
Nicotinamide adenine dinucleotide phosphate
- CNS:
-
Central nervous system
- DMSO:
-
Dimethyl sulfoxide
- DMED:
-
Dulbecco’s Modified Eagle Medium
- EDTA:
-
Ethylenediamine tetraacetic acid
- ELISA:
-
Enzyme-linked immunosorbent assay
- SASA:
-
Students Against Substance Abuse
- FOSA:
-
Fiber Optic Sensing Association
- FISA:
-
Foreign Intelligence Surveillance Act of 1978
- NRU:
-
Neutral red uptake
References
Priani, S. E., & Fakih, T. M. (2021). Insights into molecular interaction of flavonoid compounds in citrus peel bound to collagenase and elastase enzymes: A computational study. Pharmaceutical Sciences and Research, 8, 5.
Ghimeray, A. K., Jung, U. S., Lee, H. Y., Kim, Y. H., Ryu, E. K., & Chang, M. S. (2015). In vitro antioxidant, collagenase inhibition, and in vivo anti-wrinkle effects of combined formulation containing Punica granatum, Ginkgo biloba, Ficus carica, and Morus alba fruits extract. Clinical, Cosmetic and Investigational Dermatology, 8, 389–396.
Boran, R., Uğur, A., & Saraç, N. (2018). Investigation of hyaluronidase, collagenase and elastase inhibitory potentials and comparative evaluation of the antimicrobial, antioxidant and homeostatic activities of two natural polysaccharides. Süleyman Demirel Üniversitesi Fen Bilimleri Enstitüsü Dergisi, 22, 1182–1189.
Abdul Wahab, N., Abdul Rahman, R., Ismail, A., Mustafa, S., & Hashim, P. (2014). Assessment of antioxidant capacity, anti-collagenase and anti-elastase assays of Malaysian unfermented cocoa bean for cosmetic application. Natural Products Chemistry & Research, 2(3), 1–6.
Oshima, N., Narukawa, Y., Takeda, T., & Kiuchi, F. (2013). Collagenase inhibitors from Viola yedoensis. The Journal of Natural Medicines, 67, 240–245.
Hong, Y. H., Jung, E. Y., Noh, D. O., & Suh, H. J. (2014). Physiological effects of formulation containing tannase-converted green tea extract on skin care: Physical stability, collagenase, elastase, and tyrosinase activities. Integrative Medicine Research, 3, 25–33.
Wittenauer, J., Mäckle, S., Sußmann, D., Schweiggert-weisz, U., & Carle, R. (2015). Inhibitory effects of polyphenols from grape pomace extract on collagenase and elastase activity. Fitoterapia, 101, 179–187.
Han, X., Zhu, X., Hong, Z., Wei, L., Ren, Y., Wan, F., Zhu, S., Peng, H., Guo, L., Rao, L., & Feng, L. (2017). Structure-based rational design of novel inhibitors against fructose-1,6-bisphosphate aldolase from Candida albicans. Journal of Chemical Information and Modeling, 57(6), 1426–1438.
Jiang, T., Che, Q., Lin, Y., Li, H., & Zhang, N. (2006). Aldose reductase regulates TGF-beta1-induced production of fibronectin and type IV collagen in cultured rat mesangial cells. Nephrology, 11(2), 105–112.
Pal, P. B., Sonowal, H., Shukla, K., Srivastava, S. K., & Ramana, K. V. (2017). Aldose reductase mediates NLRP3 inflammasome-initiated innate immune response in hyperglycemia-induced Thp1 monocytes and male mice. Endocrinology, 158(10), 3661–3675.
Wei, J., Zhang, Y., Luo, Y., et al. (2014). Aldose reductase regulates miR-200a-3p/141-3p to coordinate Keap1–Nrf2, Tgfβ1/2, and Zeb1/2 signaling in renal mesangial cells and the renal cortex of diabetic mice. Free Radical Biology and Medicine, 67, 91–102.
Huang, Z., Hong, Q., Zhang, X., et al. (2017). Aldose reductase mediates endothelial cell dysfunction induced by high uric acid concentrations. Cell Communication and Signaling, 15(1), 3.
Dunlop, M. (2000). Aldose reductase and the role of the polyol pathway in diabetic nephropathy. Kidney International, 58, S3–S12.
He, J., Gao, H.-X., Yang, N., Zhu, X. D., Sun, R. B., Xie, Y., Zeng, C. H., Zhang, J. W., Wang, J. K., Ding, F., & Aa, J. Y. (2019). The aldose reductase inhibitor epalrestat exerts nephritic protection on diabetic nephropathy in db/db mice through metabolic modulation. Acta Pharmacologica Sinica, 40(1), 86–97.
Quattrini, L., & La Motta, C. (2019). Aldose reductase inhibitors: 2013-present. Expert Opinion on Therapeutic Patents, 29, 199–213.
Prnova, M. S., Kovacikova, L., Svik, K., Bezek, S., Elmazoğlu, Z., Karasu, C., & Stefek, M. (2020). Triglyceride-lowering effect of the aldose reductase inhibitor cemtirestat-another factor that may contribute to attenuation of symptoms of peripheral neuropathy in STZ-diabetic rats. Naunyn-Schmiedeberg’s Archives of Pharmacology, 393, 651–661.
Kansal, R. (2016). Acute myeloid leukemia in the era of precision medicine: Recent advances in diagnostic classification and risk stratification. Cancer Biology & Medicine, 13, 41–54.
De Kouchkovsky, I., & Abdul-Hay, M. (2016). Acute myeloid leukemia: A comprehensive review and 2016 update. Blood Cancer Journal, 6, e441. https://doi.org/10.1038/bcj.2016.50
Redaelli, A., Laskin, B. L., Stephens, J. M., Botteman, M. F., & Pashos, C. L. (2005). A systematic literature review of the clinical and epidemiological burden of acute lymphoblastic leukaemia (ALL). European Journal of Cancer Care, 14(1), 53–62.
Paul, S., Kantarjian, H., & Jabbour, E. J. (2016). Adult acute lymphoblastic leukemia. Mayo Clinic Proceedings, 91(11), 1645–1666.
Timms, J. A., et al. (2016). DNA methylation as a potential mediator of environmental risks in the development of childhood acute lymphoblastic leukemia. Epigenomics, 8(4), 519–536.
Siegel, R. L., Miller, K. D., & Jemal, A. (2016). Cancer statistics, 2016. CA: A Cancer Journal for Clinicians, 66(1), 7–30.
Grimwade, D., & Hills, R. K. (2009). Independent prognostic factors for AML outcome. Hematology, 2009, 385–395.
Tüzün, B., & Saripinar, E. (2020). Molecular docking and 4D-QSAR model of methanone derivatives by electron conformational-genetic algorithm method. Journal of the Iranian Chemical Society, 17, 985–1000.
Akkoç, S., Tüzün, B., İlhan, İÖ., & Akkurt, M. (2020). Investigation of structural, spectral, electronic, and biological properties of 1,3-disubstituted benzimidazole derivatives. Journal of Molecular Structure, 1219, 128582.
Genç Bilgiçli, H., Bilgiçli, A. T., Günsel, A., Tüzün, B., Ergön, D., Yarasir, M. N., & Zengin, M. (2020). Turn-on fluorescent probe for Zn2+ ions based on thiazolidine derivative. Applied Organometallic Chemistry, 34(6), e5624.
Douche, D., Elmsellem, H., Guo, L., Hafez, B., Tüzün, B., El Louzi, A., Bougrina, K., Karrouchi, K., & Himmi, B. (2020). Anti-corrosion performance of 8-hydroxyquinoline derivatives for mild steel in acidic medium: Gravimetric, electrochemical, DFT and molecular dynamics simulation investigations. Journal of Molecular Liquids, 308, 113042.
Dilshad, R., Khan, K. R., Ahmad, S., Aati, H. Y., Al-qahtani, J. H., Sherif, A. E., Hussain, M., Ghalloo, B. A., Tahir, H., Basit, A., & Ahmed, M. (2022). Phytochemical profiling, in vitro biological activities, and in-silico molecular docking studies of Typha domingensis. Arabian Journal of Chemistry, 15(10), 104133.
Wang, L., Lee, W., Oh, J. Y., Cui, Y. R., Ryu, B., & Jeon, Y. J. (2018). Protective effect of sulfated polysaccharides from Celluclast-assisted extract of Hizikia fusiforme against ultraviolet B-induced skin damage by regulating NF-κB, AP-1, and MAPKs signaling pathways in vitro in human dermal fibroblasts. Marine Drugs, 16(7), 239.
Barla, F., Higashijima, H., Funai, S., Sugimoto, K., Harada, N., Yamaji, R., Fujita, T., & Nakano, Y. H. (2009). Inui Inhibitive effects of alkyl gallates on hyaluronidase and collagenase. Bioscience, Biotechnology, and Biochemistry, 73, 2335–2337.
Zeng, Z. S., Cohen, A. M., & Guillem, J. G. (1999). Loss of basement membrane type IV collagen is associated with increased expression of metalloproteinases 2 and 9 (MMP-2 and MMP-9) during human colorectal tumorigenesis. Carcinogenesis, 20, 749–755.
Gamal, H., & Munusamy, S. (2016). Aldose reductase as a drug target for treatment of diabetic nephropathy: Promises and challenges. Protein & Peptide Letters, 24(1), 71–77.
Iso, K., Tada, H., Kuboki, K., & Inokuchi, T. (2001). Long-term effect of epalrestat, an aldose reductase inhibitor, on the development of incipient diabetic nephropathy in type 2 diabetic patients. Journal of Diabetes and Its Complications, 15(5), 241–244.
Srivastava, S. K., Yadav, U. C. S., Reddy, A. B. M., et al. (2011). Aldose reductase inhibition suppresses oxidative stress-induced inflammatory disorders. Chemico-Biological Interactions, 191(1–3), 330–338.
Schrodinger, L. (2019). Small-molecule drug discovery suite 2019-4
Schrödinger Release 2019-4: Protein preparation wizard; Epik, Schrödinger, LLC, New York, NY, 2016; Impact, Schrödinger, LLC, New York, NY, 2016; Prime, Schrödinger, LLC, New York, NY, 2019.
Friesner, R. A., Murphy, R. B., Repasky, M. P., Frye, L. L., Greenwood, J. R., Halgren, T. A., Sanschagrin, P. C., & Mainz, D. T. (2006). Extra precision glide: Docking and scoring incorporating a model of hydrophobic enclosure for protein−ligand complexes. Journal of Medicinal Chemistry, 49, 6177–6196.
Sastry, G. M., Adzhigirey, M., Day, T., Annabhimoju, R., & Sherman, W. (2013). Protein and ligand preparation: Parameters, protocols, and influence on virtual screening enrichments. Journal of Computer-Aided Molecular Design, 27(3), 221–234.
Schrödinger, LLC. (2019). Schrödinger release 2019-4: LigPrep. Schrödinger, LLC.
Shaneza, A., Umesh Kumar, G., Deepa, S., & Tahseen, K. (2018). Herbal treatment for the ovarian cancer SGVU. Journal of Pharmaceutical Education and Research, 3(2), 325–329.
Li, Z.-L., Fan, C., Chen, S.-L., Song, Y.-F., Yang, Y.-J., & Wang, S.-J. (2014). Synthesis of darirestat as an aldose reductase inhibitor (in Chinese). Journal of Shenyang Pharmaceutical University, 31(7), 521–525.
Grewal, A. S., Bhardwaj, S., Pandita, D., Lather, V., & Sekhon, B. S. (2015). Updates on aldose reductase inhibitors for management of diabetic complications and non-diabetic diseases. Mini-Reviews in Medicinal Chemistry, 16(2), 120–162.
Peter, J. (2010). Oates: Aldose reductase inhibitors and diabetic kidney disease. Current Opinion in Investigational Drugs, 11(4), 402–416.
Thring, T. S., Hili, P., & Naughton, D. P. (2009). Anti-collagenase, anti-elastase and anti-oxidant activities of extracts from 21 plants. BMC Complementary and Alternative Medicine, 9, 27.
Bauman, L. (2004). CosmoDerm/CosmoPlast (human bioengineered collagen) for the aging face. Facial Plastic Surgery, 20, 125–128.
Chatatikun, M., & Chiabchalard, A. (2017). Thai plants with high antioxidant levels, free radical scavenging activity, anti-tyrosinase and anti-collagenase activity. BMC Complementary and Alternative Medicine, 17, 487.
Du, Q., Qian, Y., Yao, X., & Xue, W. (2020). Elucidating the tight-binding mechanism of two oral anticoagulants to factor Xa by using induced-fit docking and molecular dynamics simulation. Journal of Biomolecular Structure and Dynamics, 38(2), 625–633.
Schrödinger, LLC. (2020). Schrödinger release 2020-1: QikProp. Schrödinger, LLC.
Türkmenoğlu, B., & Güzel, Y. (2018). Molecular docking and 4D-QSAR studies of metastatic cancer inhibitor thiazoles. Computational Biology and Chemistry, 76, 327–337.
Zheng, X., Zhang, L., Chen, W., Chen, Y., Xie, W., & Hu, X. (2012). Partial inhibition of aldose reductase by nitazoxanide and its molecular basis. ChemMedChem, 7(11), 1921–1923.
Xu, Q., He, C., Xiao, C., & Chen, X. (2016). Reactive oxygen species (ROS) responsive polymers for biomedical applications. Macromolecular Bioscience, 16, 635–646.
Zhang, H., Xiong, H., Ahmed, W., Yao, Y., Wang, S., Fan, C., & Gao, C. (2021). Reactive oxygen species-responsive and scavenging polyurethane nanoparticles for treatment of osteoarthritis in vivo. Chemical Engineering Journal, 409, 128147.
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
YC contributed to the study conception and design. Material preparation, data collection and analysis were performed by JX. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors have no relevant financial or non-financial interests to disclose.
Ethical Approval
Not applicable.
Consent to Participate
Not applicable.
Consent to Publish
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chu, Y., Xiao, J. 2′-Hydroxy-4′,5′-dimethoxyacetophenone Exhibit Collagenase, Aldose Reductase Inhibition, and Anticancer Activity Against Human Leukemic Cells: An In Vitro, and In Silico Study. Mol Biotechnol 65, 881–890 (2023). https://doi.org/10.1007/s12033-022-00588-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12033-022-00588-9