Abstract
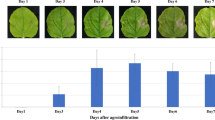
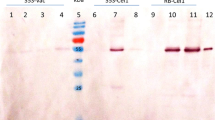
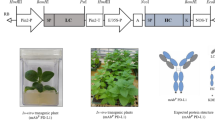
Plants are promising drug-production platforms with high economic efficiency, stability, and convenience in mass production. However, studies comparing the equivalency between the original antibodies and those produced in plants are limited. Amino acid sequences that constitute the Fab region of an antibody are diverse, and the post-transcriptional modifications that occur according to these sequences in animals and plants are also highly variable. In this study, rituximab, a blockbuster antibody drug used in the treatment of non-Hodgkin’s lymphoma, was produced in Nicotiana benthamiana leaves and Arabidopsis thaliana callus, and was compared to the original rituximab produced in CHO cells. Interestingly, the epitope recognition and antigen-binding abilities of rituximab from N. benthamiana leaves were almost lost. In the case of rituximab produced in A. thaliana callus, the specific binding ability and CD20 capping activity were maintained, but the binding affinity was less than 50% of that of original rituximab from CHO cells. These results suggest that different plant species exhibit different binding affinities. Accordingly, in addition to the differences in PTMs between mammals and plants, the differences between the species must also be considered in the process of producing antibodies in plants.






Similar content being viewed by others
References
Hiatt, A., Cafferkey, R., & Bowdish, K. (1989). Production of antibodies in transgenic plants. Nature, 342, 76–78.
Ullrich, K. K., Hiss, M., & Rensing, S. A. (2015). Means to optimize protein expression in transgenic plants. Current Opinion in Biotechnology, 32, 61–67.
Twyman, R. M., Stoger, E., Schillberg, S., Christou, P., & Fischer, R. (2003). Molecular farming in plants: Host systems and expression technology. Trends in Biotechnology, 21, 570–578.
Lomonossoff, G. P., & D’Aoust, M. A. (2016). Plant-produced biopharmaceuticals: A case of technical developments driving clinical deployment. Science, 353, 1237–1240.
Shaaltiel, Y., Bartfeld, D., Hashmueli, S., Baum, G., Brill-Almon, E., Galili, G., Dym, O., Boldin-Adamsky, S. A., Silman, I., Sussman, J. L., Futerman, A. H., & Aviezer, D. (2007). Production of glucocerebrosidase with terminal mannose glycans for enzyme replacement therapy of Gaucher’s disease using a plant cell system. Plant Biotechnology Journal, 5, 579–590.
Fischer, R., & Buyel, J. F. (2020). Molecular farming - The slope of enlightenment. Biotechnology Advances, 40, 107519.
Gomord, V., & Faye, L. (2004). Posttranslational modification of therapeutic proteins in plants. Current Opinion in Plant Biology, 7, 171–181.
Murin, C. D., Fusco, M. L., Bornholdt, Z. A., Qiu, X., Olinger, G. G., Zeitlin, L., Kobinger, G. P., Ward, A. B., & Saphire, E. O. (2014). Structures of protective antibodies reveal sites of vulnerability on Ebola virus. Proceedings of the National Academy of Science U S A, 111, 17182–17187.
Zeitlin, L., Pettitt, J., Scully, C., Bohorova, N., Kim, D., Pauly, M., Hiatt, A., Ngo, L., Steinkellner, H., Whaley, K. J., & Olinger, G. G. (2011). Enhanced potency of a fucose-free monoclonal antibody being developed as an Ebola virus immunoprotectant. Proceedings of the National Academy of Science U S A, 108, 20690–20694.
Herter, S., Herting, F., Mundigl, O., Waldhauer, I., Weinzierl, T., Fauti, T., Muth, G., Ziegler-Landesberger, D., Van Puijenbroek, E., Lang, S., Duong, M. N., Reslan, L., Gerdes, C. A., Friess, T., Baer, U., Burtscher, H., Weidner, M., Dumontet, C., Umana, P., … Klein, C. (2013). Preclinical activity of the type II CD20 antibody GA101 (obinutuzumab) compared with rituximab and ofatumumab in vitro and in xenograft models. Molecular Cancer Therapeutics, 12, 2031–2042.
Strasser, R., Altmann, F., & Steinkellner, H. (2014). Controlled glycosylation of plant-produced recombinant proteins. Current Opinion in Biotechnology, 30, 95–100.
Strasser, R., Stadlmann, J., Schahs, M., Stiegler, G., Quendler, H., Mach, L., Glossl, J., Weterings, K., Pabst, M., & Steinkellner, H. (2008). Generation of glyco-engineered Nicotiana benthamiana for the production of monoclonal antibodies with a homogeneous human-like N-glycan structure. Plant Biotechnology Journal, 6, 392–402.
Reff, M. E., Carner, K., Chambers, K. S., Chinn, P. C., Leonard, J. E., Raab, R., Newman, R. A., Hanna, N., & Anderson, D. R. (1994). Depletion of B cells in vivo by a chimeric mouse human monoclonal antibody to CD20. Blood, 83, 435–445.
Freeman, C. L., & Sehn, L. H. (2018). A tale of two antibodies: Obinutuzumab versus rituximab. British Journal of Haematology, 182, 29–45.
Salles, G., Barrett, M., Foà, R., Maurer, J., O’Brien, S., Valente, N., Wenger, M., & Maloney, D. G. (2017). Rituximab in B-cell hematologic malignancies: A review of 20 years of clinical experience. Advances in Therapy, 34, 2232–2273.
Smith, M. R. (2003). Rituximab (monoclonal anti-CD20 antibody): Mechanisms of action and resistance. Oncogene, 22, 7359–7368.
Glennie, M. J., French, R. R., Cragg, M. S., & Taylor, R. P. (2007). Mechanisms of killing by anti-CD20 monoclonal antibodies. Molecular Immunology, 44, 3823–3837.
Rogers, L. M., Veeramani, S., & Weiner, G. J. (2014). Complement in monoclonal antibody therapy of cancer. Immunologic Research, 59, 203–210.
Bardor, M., Faveeuw, C., Fitchette, A. C., Gilbert, D., Galas, L., Trottein, F., Faye, L., & Lerouge, P. (2003). Immunoreactivity in mammals of two typical plant glyco-epitopes, core alpha (1,3)-fucose and core xylose. Glycobiology, 13, 427–434.
Shaaltiel, Y., & Tekoah, Y. (2016). Plant specific N-glycans do not have proven adverse effects in humans. Nature Biotechnology, 34, 706–708.
Ma, J. K., Drossard, J., Lewis, D., Altmann, F., Boyle, J., Christou, P., Cole, T., Dale, P., van Dolleweerd, C. J., Isitt, V., Katinger, D., Lobedan, M., Mertens, H., Paul, M. J., Rademacher, T., Sack, M., Hundleby, P. A., Stiegler, G., Stoger, E., … Fischer, R. (2015). Regulatory approval and a first-in-human phase I clinical trial of a monoclonal antibody produced in transgenic tobacco plants. Plant Biotechnology Journal, 13, 1106–1120.
Spoel, S. H. (2018). Orchestrating the proteome with post-translational modifications. Journal of Experimental Botany, 69, 4499–4503.
Arnold, J. N., Wormald, M. R., Sim, R. B., Rudd, P. M., & Dwek, R. A. (2007). The impact of glycosylation on the biological function and structure of human immunoglobulins. Annual Review of Immunology, 25, 21–50.
Rudd, P. M., Elliott, T., Cresswell, P., Wilson, I. A., & Dwek, R. A. (2001). Glycosylation and the immune system. Science, 291, 2370–2376.
Castilho, A., Windwarder, M., Gattinger, P., Mach, L., Strasser, R., Altmann, F., & Steinkellner, H. (2014). Proteolytic and N-glycan processing of human α1-antitrypsin expressed in Nicotiana benthamiana. Plant Physiology, 166, 1839–1851.
Walsh, G., & Jefferis, R. (2006). Post-translational modifications in the context of therapeutic proteins. Nature Biotechnology, 24, 1241–1252.
Strasser, R. (2012). Challenges in O-glycan engineering of plants. Frontiers in Plant Science, 3, 218.
Lee, J. W., Heo, W., Lee, J., Jin, N., Yoon, S. M., Park, K. Y., Kim, E. Y., Kim, W. T., & Kim, J. Y. (2018). The B cell death function of obinutuzumab-HDEL produced in plant (Nicotiana benthamiana L.) is equivalent to obinutuzumab produced in CHO cells. PLoS ONE, 13, e0191075.
Harrison, S. J., Mott, E. K., Parsley, K., Aspinall, S., Gray, J. C., & Cottage, A. (2006). A rapid and robust method of identifying transformed Arabidopsis thaliana seedlings following floral dip transformation. Plant Methods, 2, 19.
Doelling, J. H., & Pikaard, C. S. (1993). Transient expression in Arabidopsis thaliana protoplasts derived from rapidly established cell suspension cultures. Plant Cell Reports, 12, 241–244.
Lauber, M. A., Yu, Y.-Q., Brousmiche, D. W., Hua, Z., Koza, S. M., Magnelli, P., Guthrie, E., Taron, C. H., & Fountain, K. J. (2015). Rapid preparation of released N-glycans for HILIC analysis using a labeling reagent that facilitates sensitive fluorescence and ESI-MS detection. Analytical Chemistry, 87, 5401–5409.
Kommineni, V., Markert, M., Ren, Z., Palle, S., Carrillo, B., Deng, J., Tejeda, A., Nandi, S., McDonald, K. A., Marcel, S., & Holtz, B. (2019). In vivo glycan engineering via the mannosidase I Inhibitor (Kifunensine) improves efficacy of rituximab manufactured in nicotiana benthamiana plants. International Journal of Molecular Sciences, 20(1), 194.
Triguero, A., Cabrera, G., Cremata, J. A., Yuen, C. T., Wheeler, J., & Ramirez, N. I. (2005). Plant-derived mouse IgG monoclonal antibody fused to KDEL endoplasmic reticulum-retention signal is N-glycosylated homogeneously throughout the plant with mostly high-mannose-type N-glycans. Plant Biotechnology Journal, 3, 449–457.
Conrad, U., & Fiedler, U. (1998). Compartment-specific accumulation of recombinant immunoglobulins in plant cells: An essential tool for antibody production and immunomodulation of physiological functions and pathogen activity. Plant Molecular Biology, 38, 101–109.
Abraham, R., Moller, D., Gabel, D., Senter, P., Hellström, I., & Hellström, K. E. (1991). The influence of periodate oxidation on monoclonal antibody avidity and immunoreactivity. Journal of Immunological Methods, 144, 77–86.
Bennett, L. D., Yang, Q., Berquist, B. R., Giddens, J. P., Ren, Z., Kommineni, V., Murray, R. P., White, E. L., Holtz, B. R., Wang, L. X., & Marcel, S. (2018). Implementation of glycan remodeling to plant-made therapeutic antibodies. International Journal of Molecular Sciences, 19(2), 421.
Zhao, F., Yu, C. H., & Liu, Y. (2017). Codon usage regulates protein structure and function by affecting translation elongation speed in Drosophila cells. Nucleic Acids Research, 45, 8484–8492.
Yu, M., Brown, D., Reed, C., Chung, S., Lutman, J., Stefanich, E., Wong, A., Stephan, J. P., & Bayer, R. (2012). Production, characterization, and pharmacokinetic properties of antibodies with N-linked mannose-5 glycans. MAbs, 4, 475–487.
Acknowledgements
This work was supported by Grants from the National Research Foundation of Korea, Project Nos. NFR-2019R1A2C1086348 to J.Y.K.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declared that no competing interests exist.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kang, C.E., Lee, S., Seo, D.H. et al. Comparison of CD20 Binding Affinities of Rituximab Produced in Nicotiana benthamiana Leaves and Arabidopsis thaliana Callus. Mol Biotechnol 63, 1016–1029 (2021). https://doi.org/10.1007/s12033-021-00360-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12033-021-00360-5