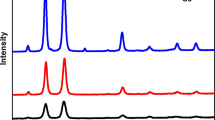
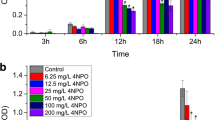
Abstract
Cell density-based intercellular signaling mechanism is known as Quorum sensing (QS); it serves a significant role in regulating the pathogenic factors. The objective of the present study was to assess the influence of chitosan-zinc oxide nanocomposite (CH-ZnO nanocomposite), alone and in combination with gentamicin, on the sensitivity to hydrogen peroxide (H2O2), the production of pathogenic factors and QS-regulated genes of Pseudomonas aeruginosa. The efficacy of the minimum inhibitory concentration (MIC) and 1/4 MIC of the CH-ZnO nanocomposite, alone and in combination with gentamicin, on the sensitivity to H2O2, pyocyanin secretion, swarming and twitching motilities was evaluated. In addition, the expression of some QS-regulated genes including rhlI, rhlR, lasI and lasR genes was measured by Real-time quantitative PCR (RT-qPCR) following exposure to the nanocomposite. The results demonstrated that at MIC concentrations, the gentamicin-loaded CH-ZnO nanocomposite significantly inhibited QS-regulated phenotypes such as pyocyanin secretion (82.4%), swarming (76%) and twitching (73.6%) motilities; further it increased the inhibition growth zone (134.5%), as well as, at 1/4 MIC concentration decreased the expression of lasI (72%), lasR (78%), rhlI (76%) and rhlR (82%) genes; as compared to untreated P. aeruginosa PAO1 (P < 0.05). Our results also demonstrated that the CH-ZnO nanocomposite combined with gentamicin could be a potential innovative candidate, which could be broadly applied in the treatment of P. aeruginosa infections.








Similar content being viewed by others
References
Matta, R., Hallit, S., Hallit, R., Bawab, W., Rogues, A.-M., & Salameh, P. (2018). Epidemiology and microbiological profile comparison between community and hospital acquired infections: a multicenter retrospective study in Lebanon. Journal of Infection and Public Health, 11, 405–411.
Tuon, F. F., Gortz, L. W., & Rocha, J. L. (2012). Risk factors for pan-resistant Pseudomonas aeruginosa bacteremia and the adequacy of antibiotic therapy. The Brazilian Journal of Infectious Diseases, 16, 351–356.
Pelgrift, R. Y., & Friedman, A. J. (2013). Nanotechnology as a therapeutic tool to combat microbial resistance. Adv. Drug Del. Rev., 65, 1803–1815.
Hemmati, F., Salehi, R., Ghotaslou, R., Kafil, H. S., Hasani, A., Gholizadeh, P., Nouri, R., & Rezaee, M. A. (2020). Quorum quenching: a potential target for antipseudomonal therapy. Infection and Drug Resistance, 13, 2989.
Li, S., Chen, S., Fan, J., Cao, Z., Ouyang, W., Tong, N., Hu, X., Hu, J., Li, P., & Feng, Z. (2018). Anti-biofilm effect of novel thiazole acid analogs against Pseudomonas aeruginosa through IQS pathways. European Journal of Medicinal Chemistry, 145, 64–73.
Rutherford, S. T., & Bassler, B. L. (2012). Bacterial quorum sensing: its role in virulence and possibilities for its control. Cold Spring Harbor Perspectives in Medicine, 2, 012427.
Pérez-Pérez, M., Jorge, P., Pérez Rodríguez, G., Pereira, M. O., & Lourenço, A. (2017). Quorum sensing inhibition in Pseudomonas aeruginosa biofilms: new insights through network mining. Biofouling, 33, 128–142.
LaSarre, B., & Federle, M. J. (2013). Exploiting quorum sensing to confuse bacterial pathogens. Microbiology and molecular biology reviews, 77, 73–111.
Kalia, V. C. (2013). Quorum sensing inhibitors: an overview. Biotechnology Advances, 31, 224–245.
Starkey, M., Lepine, F., Maura, D., Bandyopadhaya, A., Lesic, B., He, J., Kitao, T., Righi, V., Milot, S., & Tzika, A. (2014). Identification of anti-virulence compounds that disrupt quorum-sensing regulated acute and persistent pathogenicity. PLoS Pathogens, 10, 1004321.
Liu, X., Ma, L., Mao, Z. and Gao, C. (2011) Chitosan-based biomaterials for tissue repair and regeneration, in Chitosan for Biomaterials II, Springer: pp. 81–127.
Ebrahimzadeh, S., Bari, M. R., Hamishehkar, H., Kafil, H. S., & Lim, L. T. (2021). Essential oils-loaded electrospun chitosan-poly (vinyl alcohol) nonwovens laminated on chitosan film as bilayer bioactive edible films. LWT, 144, 111217.
Aranaz, I., Harris, R., & Heras, A. (2010). Chitosan amphiphilic derivatives. Chemistry and applications. Curr. Org. Chem., 14, 308–330.
Souza, M. P., Vaz, A. F., Correia, M. T., Cerqueira, M. A., Vicente, A. A., & Carneiro-da-Cunha, M. G. (2014). Quercetin-loaded lecithin/chitosan nanoparticles for functional food applications. Food and bioprocess technology, 7, 1149–1159.
Hemmati, F., Rezaee, M. A., Ebrahimzadeh, S., Yousefi, L., Nouri, R., Kafil, H. S., & Gholizadeh, P. (2021). Novel Strategies to Combat Bacterial Biofilms. Mol: Biotechnol. https://doi.org/10.1007/s12033-021-00325-8
Hemmati, F., Salehi, R., Ghotaslou, R., Kafil, H. S., Hasani, A., Gholizadeh, P., & Rezaee, M. A. (2020). The assessment of antibiofilm activity of chitosan-zinc oxide-gentamicin nanocomposite on Pseudomonas aeruginosa and Staphylococcus aureus. International Journal of Biological Macromolecules, 163, 2248–2258.
Ong, S.-Y., Wu, J., Moochhala, S. M., Tan, M.-H., & Lu, J. (2008). Development of a chitosan-based wound dressing with improved hemostatic and antimicrobial properties. Biomaterials, 29, 4323–4332.
Ma, Z., Garrido-Maestu, A., & Jeong, K. C. (2017). Application, mode of action, and in vivo activity of chitosan and its micro-and nanoparticles as antimicrobial agents: A review. Carbohydrate Polymers, 176, 257–265.
Qin, X., Kräft, T., & Goycoolea, F. M. (2018). Chitosan encapsulation modulates the effect of trans-cinnamaldehyde on AHL-regulated quorum sensing activity. Colloids and Surfaces. B, Biointerfaces, 169, 453–461.
O’Callaghan, K. A., & Kerry, J. P. (2016). Preparation of low-and medium-molecular weight chitosan nanoparticles and their antimicrobial evaluation against a panel of microorganisms, including cheese-derived cultures. Food Control, 69, 256–261.
Muslim, S. N., Kadmy, I. M. A., Ali, A. N. M., Salman, B. K., Ahmad, M., Khazaal, S. S., Hussein, N. H., & Muslim, S. N. (2018). Chitosan extracted from Aspergillus flavus shows synergistic effect, eases quorum sensing mediated virulence factors and biofilm against nosocomial pathogen Pseudomonas aeruginosa. International Journal of Biological Macromolecules, 107, 52–58.
Segets, D., Gradl, J., Taylor, R. K., Vassilev, V., & Peukert, W. (2009). Analysis of optical absorbance spectra for the determination of ZnO nanoparticle size distribution, solubility, and surface energy. ACS Nano, 3, 1703–1710.
Reddy, K. M., Feris, K., Bell, J., Wingett, D. G., Hanley, C., & Punnoose, A. (2007). Selective toxicity of zinc oxide nanoparticles to prokaryotic and eukaryotic systems. Applied Physics Letter, 90, 213902.
Vincent, M. G., John, N. P., Narayanan, P., Vani, C., & Murugan, S. (2014). In vitro study on the efficacy of zinc oxide and titanium dioxide nanoparticles against metallo beta-lactamase and biofilm producing Pseudomonas aeruginosa. Journal of Applied Pharmaceutical Science, 4, 41.
Jafarirad, S., Mehrabi, M., Divband, B., & Kosari-Nasab, M. (2016). Biofabrication of zinc oxide nanoparticles using fruit extract of Rosa canina and their toxic potential against bacteria: a mechanistic approach. Materials Science and Engineering: C, 59, 296–302.
Voicu, G., Oprea, O., Vasile, B., & Andronescu, E. (2013). Antibacterial activity of zinc oxide-gentamicin hybrid material. Digest Journal of Nanomaterials & Biostructures (DJNB), 8, 1191–1203.
Al-Tayyar, N. A., Youssef, A. M., & Al-Hindi, R. (2020). Antimicrobial food packaging based on sustainable Bio-based materials for reducing foodborne Pathogens: A review. Food Chemistry, 310, 125915.
Vasile, B. S., Oprea, O., Voicu, G., Ficai, A., Andronescu, E., Teodorescu, A., & Holban, A. (2014). Synthesis and characterization of a novel controlled release zinc oxide/gentamicin–chitosan composite with potential applications in wounds care. International Journal of Pharmaceutics, 463, 161–169.
Ebrahimzadeh, S., Ghanbarzadeh, B., & Hamishehkar, H. (2016). Physical properties of carboxymethyl cellulose based nano-biocomposites with Graphene nano-platelets. International Journal of Biological Macromolecules, 84, 16–23.
Bala, A., Kumar, R., & Harjai, K. (2011). Inhibition of quorum sensing in Pseudomonas aeruginosa by azithromycin and its effectiveness in urinary tract infections. Journal of Medical Microbiology, 60, 300–306.
Rashid, M. H., & Kornberg, A. (2000). Inorganic polyphosphate is needed for swimming, swarming, and twitching motilities of Pseudomonas aeruginosa. Proceedings of the National Academy of Sciences, 97, 4885–4890.
Heidari, A., Haghi, F., Noshiranzadeh, N., & Bikas, R. (2017). (S, E)-2-hydroxy-N-(2-hydroxy-5-nitrobenzylidene) propane hydrazide as a quorum sensing inhibitor of Pseudomonas aeruginosa. Medicinal Chemistry Research, 26, 1947–1955.
He, X., Hwang, H.-M., Aker, W. G., Wang, P., Lin, Y., Jiang, X., & He, X. (2014). Synergistic combination of marine oligosaccharides and azithromycin against Pseudomonas aeruginosa. Microbiological Research, 169, 759–767.
Movahedi, Z., Pourakbari, B., Mahmoudi, S., Sabouni, F., ASHTIAnI, M. H., Sadeghi, R. H., & Mamishi, S. (2013). Pseudomonas aeruginosa infection among cystic fibrosis and ICU patients in the referral children medical hospital in Tehran. Iran. J. Prev. Med. Hyg., 54, 24.
Hong, D. J., Bae, I. K., Jang, I.-H., Jeong, S. H., Kang, H.-K., & Lee, K. (2015). Epidemiology and characteristics of metallo-β-lactamase-producing Pseudomonas aeruginosa. Infection & Chemotherapy, 47, 81–97.
Yang, Y.-X., Xu, Z.-H., Zhang, Y.-Q., Tian, J., Weng, L.-X., & Wang, L.-H. (2012). A new quorum-sensing inhibitor attenuates virulence and decreases antibiotic resistance in Pseudomonas aeruginosa. Journal of Microbiology, 50, 987–993.
Gholizadeh, P., Maftoon, H., Aghazadeh, M., Asgharzadeh, M., & Kafil, H. S. (2017). Current opinions in the infection control of carbapenem-resistant Enterobacteriaceae species and Pseudomonas aeruginosa. Reviews Medical Microbiology, 28, 97–103.
Darvishi, S., Javanbakht, S., Heydari, A., Kazeminava, F., Gholizadeh, P., Mahdipour, M., & Shaabani, A. (2021). Ultrasound-assisted synthesis of MIL-88(Fe) coordinated to carboxymethyl cellulose fibers: A safe carrier for highly sustained release of tetracycline. International Journal of Biological Macromolecules, 181, 937–944.
El-Mowafy, S. A., Abd El Galil, K. H., El-Messery, S. M., & Shaaban, M. I. (2014). Aspirin is an efficient inhibitor of quorum sensing, virulence and toxins in Pseudomonas aeruginosa. Microbial Pathogenesis, 74, 25–32.
El-Mowafy, S., Shaaban, M., & Abd El Galil, K. (2014). Sodium ascorbate as a quorum sensing inhibitor of P seudomonas aeruginosa. Journal of Applied Microbiology, 117, 1388–1399.
Horii, T., Morita, M., Muramatsu, H., Muranaka, Y., Kanno, T., & Maekawa, M. (2003). Effects of mupirocin at subinhibitory concentrations on flagella formation in Pseudomonas aeruginosa and Proteus mirabilis. Journal of Antimicrobial Chemotherapy, 51, 1175–1179.
Bagge, N., Schuster, M., Hentzer, M., Ciofu, O., Givskov, M., Greenberg, E. P., & Høiby, N. (2004). Pseudomonas aeruginosa biofilms exposed to imipenem exhibit changes in global gene expression and β-lactamase and alginate production. Antimicrobial Agents and Chemotherapy, 48, 1175–1187.
Kolayli, F., Karadenizli, A., Savli, H., Ergen, K., Hatirnaz, O., Balikci, E., Budak, F., & Vahaboglu, H. (2004). Effect of carbapenems on the transcriptional expression of the oprD, oprM and oprN genes in Pseudomonas aeruginosa. Journal of Medical Microbiology, 53, 915–920.
Fonseca, A., Extremina, C., Fonseca, A., & Sousa, J. (2004). Effect of subinhibitory concentration of piperacillin/tazobactam on Pseudomonas aeruginosa. Journal of Medical Microbiology, 53, 903–910.
Wahid, F., Yin, J.-J., Xue, D.-D., Xue, H., Lu, Y.-S., Zhong, C., & Chu, L.-Q. (2016). Synthesis and characterization of antibacterial carboxymethyl Chitosan/ZnO nanocomposite hydrogels. International Journal of Biological Macromolecules, 88, 273–279.
Karpuraranjith, M., & Thambidurai, S. (2017). Chitosan/zinc oxide-polyvinylpyrrolidone (CS/ZnO-PVP) nanocomposite for better thermal and antibacterial activity. International Journal of Biological Macromolecules, 104, 1753–1761.
Ali, S. G., Ansari, M. A., Jamal, Q. M. S., Almatroudi, A., Alzohairy, M. A., Alomary, M. N., Rehman, S., Mahadevamurthy, M., Jalal, M., & Khan, H. M. (2021). Butea monosperma seed extract mediated biosynthesis of ZnO NPs and their antibacterial, antibiofilm and anti-quorum sensing potentialities. Arabian Journal of Chemistry, 14, 103044.
Das, M. C., Sandhu, P., Gupta, P., Rudrapaul, P., De, U. C., Tribedi, P., Akhter, Y., & Bhattacharjee, S. (2016). Attenuation of Pseudomonas aeruginosa biofilm formation by Vitexin: a combinatorial study with azithromycin and gentamicin. Scientific Reports, 6, 1–13.
Gupta, P., Sarkar, A., Sandhu, P., Daware, A., Das, M., Akhter, Y., & Bhattacharjee, S. (2017). Potentiation of antibiotic against Pseudomonas aeruginosa biofilm: a study with plumbagin and gentamicin. Journal of Applied Microbiology, 123, 246–261.
Vinckx, T., Wei, Q., Matthijs, S., & Cornelis, P. (2010). The Pseudomonas aeruginosa oxidative stress regulator OxyR influences production of pyocyanin and rhamnolipids: protective role of pyocyanin. Microbiology, 156, 678–686.
Sui, S. J. H., Fedynak, A., Hsiao, W. W., Langille, M. G., & Brinkman, F. S. (2009). The association of virulence factors with genomic islands. PLoS ONE, 4, 8094.
Heidari, A., Noshiranzadeh, N., Haghi, F., & Bikas, R. (2017). Inhibition of quorum sensing related virulence factors of Pseudomonas aeruginosa by pyridoxal lactohydrazone. Microbial Pathogenesis, 112, 103–110.
Shen, L., Shi, Y., Zhang, D., Wei, J., Surette, M. G., & Duan, K. (2008). Modulation of secreted virulence factor genes by subinhibitory concentrations of antibiotics in Pseudomonas aeruginosa. The Journal of Microbiology, 46, 441–447.
Vadekeetil, A., Alexandar, V., Chhibber, S., & Harjai, K. (2016). Adjuvant effect of cranberry proanthocyanidin active fraction on antivirulent property of ciprofloxacin against Pseudomonas aeruginosa. Microbial Pathogenesis, 90, 98–103.
Chanda, S., & Rakholiya, K. (2011). Combination therapy: Synergism between natural plant extracts and antibiotics against infectious diseases. Microbiol Book Series, 1, 520–529.
Hassett, D. J., Ma, J. F., Elkins, J. G., McDermott, T. R., Ochsner, U. A., West, S. E., Huang, C. T., Fredericks, J., Burnett, S., & Stewart, P. S. (1999). Quorum sensing in Pseudomonas aeruginosa controls expression of catalase and superoxide dismutase genes and mediates biofilm susceptibility to hydrogen peroxide. Molecular Microbiology, 34, 1082–1093.
Vadekeetil, A., Chhibber, S., & Harjai, K. (2019). Efficacy of intravesical targeting of novel quorum sensing inhibitor nanoparticles against Pseudomonas aeruginosa biofilm-associated murine pyelonephritis. Journal of Drug Targeting, 27, 995–1003.
Badawy, M. S. E., Riad, O. K. M., Taher, F., & Zaki, S. A. (2020). Chitosan and chitosan-zinc oxide nanocomposite inhibit expression of LasI and RhlI genes and quorum sensing dependent virulence factors of Pseudomonas aeruginosa. International Journal of Biological Macromolecules, 149, 1109–1117.
Qi, L., Xu, Z., Jiang, X., Hu, C., & Zou, X. (2004). Preparation and antibacterial activity of chitosan nanoparticles. Carbohydrate Research, 339, 2693–2700.
Lee, J.-H., Kim, Y.-G., Cho, M. H., & Lee, J. (2014). ZnO nanoparticles inhibit Pseudomonas aeruginosa biofilm formation and virulence factor production. Microbiological Research, 169, 888–896.
García-Lara, B., Saucedo-Mora, M., Roldán-Sánchez, J., Pérez-Eretza, B., Ramasamy, M., Lee, J., Coria-Jimenez, R., Tapia, M., Varela-Guerrero, V., & García-Contreras, R. (2015). Inhibition of quorum-sensing-dependent virulence factors and biofilm formation of clinical and environmental P seudomonas aeruginosa strains by ZnO nanoparticles. Letters in Applied Microbiology, 61, 299–305.
Saleh, M. M., Refa’tLatif, A. S. H. K. A., Abbas, H. A., & Askoura, M. (2019). Zinc oxide nanoparticles inhibits quorum sensing and virulence in Pseudomonas aeruginosa. African Health Sciences, 19, 2043–2055.
Dieppois, G., Ducret, V., Caille, O., & Perron, K. (2012). The transcriptional regulator CzcR modulates antibiotic resistance and quorum sensing in Pseudomonas aeruginosa. PLoS ONE, 7, 38148.
Divya, K., Vijayan, S., George, T. K., & Jisha, M. (2017). Antimicrobial properties of chitosan nanoparticles: mode of action and factors affecting activity. Fibers and Polymers, 18, 221–230.
Acknowledgements
This work was financially supported by the Immunology Research Center, Tabriz University of Medical Sciences, Tabriz, Iran (Grant No. 97/60073).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hemmati, F., Ghotaslou, R., Salehi, R. et al. Effects of Gentamicin-Loaded Chitosan-ZnO Nanocomposite on Quorum-Sensing Regulation of Pseudomonas Aeruginosa. Mol Biotechnol 63, 746–756 (2021). https://doi.org/10.1007/s12033-021-00336-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12033-021-00336-5