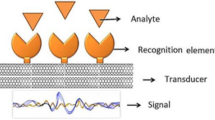
Abstract
Nanotechnology-based miniaturized devices have been a breakthrough in the pre-clinical and clinical research areas, e.g. drug delivery, personalized medicine. They have revolutionized the discovery and development of biomarker-based diagnostic devices for detection of various diseases such as tuberculosis, malaria and cancer. Nanomaterials (NMs) hold tremendous diagnostic potential due to their high surface-to-volume ratio and quantum confinement phenomenon, improving the detection limit of clinically relevant biomolecules in bio-fluids. Thus, they are helpful in the translation of bench-on platform to point-of-care (POC) screening device. The nanomaterial-based biosensor fabrication technology has also simplified and improved oral cancer (OC) or oral squamous cell carcinomas (OSCC) diagnosis. The fabrication of nano-bio sensors involves application specific modifications of NMs. The unique properties functionalized NMs have augmented their application on the nano-biosensing platform for the detection of clinically relevant biomolecules in bio-fluids. Therefore, this article summarizes the recent advancements in the process of fabrication of nano-biosensors for detection of OC.




Similar content being viewed by others
References
Mehrotra, P. (2016). Biosensors and their applications: A review. Journal of Oral Biology and Craniofacial Research. https://doi.org/10.1016/j.jobcr.2015.12.002.
Jalil, O., Pandey, C. M., & Kumar, D. (2020). Electrochemical biosensor for the epithelial cancer biomarker EpCAM based on reduced graphene oxide modified with nanostructured titanium dioxide. Microchimica Acta. https://doi.org/10.1007/s00604-020-04233-7.
Wang,C., Li, Y., Xu, E., Zhou, Q., Chen, J., Wei, W., … Liu, S. (2019). A label-free PFP-based photoelectrochemical biosensor for highly sensitive detection of PARP-1 activity. Biosensors and Bioelectronics. https://doi.org/https://doi.org/10.1016/j.bios.2019.05.013
Yadav, A. K., Dhiman, T. K., Lakshmi, G. B. V. S., Berlina, A. N., & Solanki, P. R. (2020). A highly sensitive label-free amperometric biosensor for norfloxacin detection based on chitosan-yttria nanocomposite. International Journal of Biological Macromolecules. https://doi.org/10.1016/j.ijbiomac.2020.02.089.
Rabti, A., Raouafi, A., & Raouafi, N. (2020). Chapter 13—DNA markers and nano-biosensing approaches for tuberculosis diagnosis. In P. Kesharwani (Ed.), Nanotechnology Based Approaches for Tuberculosis Treatment (pp. 207–230). London: Academic Press. https://doi.org/10.1016/B978-0-12-819811-7.00013-8.
Krampa, F. D., Aniweh, Y., Kanyong, P., & Awandare, G. A. (2020). Recent advances in the development of biosensors for malaria diagnosis. Sensors., 20, 799.
Singh, S., Singh, P. K., Umar, A., Lohia, P., Albargi, H., Castañeda, L., & Dwivedi, D. K. (2020). 2D Nanomaterial-based surface plasmon resonance sensors for biosensing applications. Micromachines. https://doi.org/10.3390/mi11080779.
Menon, K., Joy, R. A., Sood, N., & Mittal, R. (2013). The applications of bioMEMS in diagnosis, cell biology, and therapy: A review. BioNanoScience., 3, 356–366.
Wang, S., Inci, F., De Libero, G., Singhal, A., & Demirci, U. (2013). Point-of-care assays for tuberculosis: Role of nanotechnology/microfluidics. Biotechnology Advances, 31, 438–449.
Arduini, F., Cinti, S., Caratelli, V., Amendola, L., Palleschi, G., & Moscone, D. (2019). Origami multiple paper-based electrochemical biosensors for pesticide detection. Biosensors and Bioelectronics. https://doi.org/10.1016/j.bios.2018.10.0144.
Jiang, D., Ge, P., Wang, L., Jiang, H., Yang, M., Yuan, L., … Ju, X. (2019). A novel electrochemical mast cell-based paper biosensor for the rapid detection of milk allergen casein. Biosensors and Bioelectronics. https://doi.org/https://doi.org/10.1016/j.bios.2019.01.050
Park, J., Lee, W., Kim, I., Kim, M., Jo, S., Kim, W., … Park, J. (2019). Ultrasensitive detection of fibrinogen using erythrocyte membrane-draped electrochemical impedance biosensor. Sensors and Actuators B: Chemical. https://doi.org/https://doi.org/10.1016/j.snb.2019.05.016
Luo, Z., Xu, Y., Huang, Z., Chen, J., Wang, X., Li, D., … Duan, Y. (2020). A rapid, adaptative DNA biosensor based on molecular beacon-concatenated dual signal amplification strategies for ultrasensitive detection of p53 gene and cancer cells. Talanta. https://doi.org/https://doi.org/10.1016/j.talanta.2019.120638
Bu, T., Huang, Q., Yan, L., Zhang, W., Dou, L., Huang, L., … Zhang, D. (2019). Applicability of biological dye tracer in strip biosensor for ultrasensitive detection of pathogenic bacteria. Food Chemistry. https://doi.org/https://doi.org/10.1016/j.foodchem.2018.09.066
Gao, J., Huang, W., Chen, Z., Yi, C., & Jiang, L. (2019). Simultaneous detection of glucose, uric acid and cholesterol using flexible microneedle electrode array-based biosensor and multi-channel portable electrochemical analyzer. Sensors and Actuators B: Chemical. https://doi.org/10.1016/j.snb.2019.02.020.
Sin, M. L. Y., Mach, K. E., Wong, P. K., & Liao, J. C. (2014). Advances and challenges in biosensor-based diagnosis of infectious diseases. Expert Review of Molecular Diagnostics. https://doi.org/10.1586/14737159.2014.888313.
Yan, X., Yang, M., Zhang, Z., Liang, L., Wei, D., Wang, M., … Yao, J. (2019). The terahertz electromagnetically induced transparency-like metamaterials for sensitive biosensors in the detection of cancer cells. Biosensors and Bioelectronics. https://doi.org/https://doi.org/10.1016/j.bios.2018.11.014
Chakraborty, D., Viveka, T. S., Arvind, K., Shyamsundar, V., Kanchan, M., Alex, S. A., … Mukherjee, A. (2018). A facile gold nanoparticle-based ELISA system for detection of osteopontin in saliva: Towards oral cancer diagnostics. Clinica Chimica Acta. https://doi.org/https://doi.org/10.1016/j.cca.2017.09.009
Warnakulasuriya, S. (2009). Global epidemiology of oral and oropharyngeal cancer. Oral Oncology, 45(4), 309–316. https://doi.org/10.1016/j.oraloncology.2008.06.002.
Jemal, A., Bray, F., Center, M. M., Ferlay, J., Ward, E., & Forman, D. (2011). Global cancer statistics. CA: A Cancer Journal for Clinicians, 61(2), 69–90. https://doi.org/10.3322/caac.20107.
Akhtar, S., Sheikh, A. A., & Qureshi, H. U. (2012). Chewing areca nut, betel quid, oral snuff, cigarette smoking and the risk of oesophageal squamous-cell carcinoma in South Asians: A multicentre case-control study. European Journal of Cancer. https://doi.org/10.1016/j.ejca.2011.06.008.
Subapriya, R., Thangavelu, A., Mathavan, B., Ramachandran, C. R., & Nagini, S. (2007). Assessment of risk factors for oral squamous cell carcinoma in Chidambaram Southern India: A case-control study. European Journal of Cancer Prevention. https://doi.org/10.1097/01.cej.0000228402.53106.9e.
Kumar, S., Mehrotra, D., Mishra, S., Goel, M. M., Kumar, S., Mathur, P., … Pandey, C. M. (2015). Epidemiology of substance abuse in the population of Lucknow. Journal of Oral Biology and Craniofacial Research. https://doi.org/https://doi.org/10.1016/j.jobcr.2015.08.010
Mehrotra, D., Kumar, S., Mishra, S., Kumar, S., Mathur, P., Pandey, C. M., … Chaudhry, K. (2017). Pan masala habits and risk of oral precancer: A cross-sectional survey in 0.45 million people of North India. Journal of Oral Biology and Craniofacial Research. https://doi.org/https://doi.org/10.1016/j.jobcr.2016.12.003
Walitzer, K. S., Dearing, R. L., Barrick, C., & Shyhalla, K. (2015). Tobacco smoking among male and female alcohol treatment-seekers: Clinical complexities, treatment length of stay, and goal achievement. Substance Use & Misuse. https://doi.org/10.3109/10826084.2014.962050.
Wang, Z., Zhang, J., Guo, Y., Wu, X., Yang, W., Xu, L., … Fu, F. (2013). A novel electrically magnetic-controllable electrochemical biosensor for the ultra sensitive and specific detection of attomolar level oral cancer-related microRNA. Biosensors & Bioelectronics, 45:108-113. https://doi.org/https://doi.org/10.1016/j.bios.2013.02.007
Senapati, S., Slouka, Z., Shah, S. S., Behura, S. K., Shi, Z., Stack, M. S., … Chang, H. C. (2014). An ion-exchange nanomembrane sensor for detection of nucleic acids using a surface charge inversion phenomenon. Biosensors & Bioelectronics, 60:92–100. https://doi.org/https://doi.org/10.1016/j.bios.2014.04.008
Rizwan, M., Koh, D., Booth, M. A., & Ahmed, M. U. (2018). Combining a gold nanoparticle-polyethylene glycol nanocomposite and carbon nanofiber electrodes to develop a highly sensitive salivary secretory immunoglobulin A immunosensor. Sensors and Actuators B: Chemical, 255, 557–563. https://doi.org/10.1016/j.snb.2017.08.079.
Aydın, E. B., Aydın, M., & Sezgintürk, M. K. (2017). A highly sensitive immunosensor based on ITO thin films covered by a new semi-conductive conjugated polymer for the determination of TNFα in human saliva and serum samples. Biosensors and Bioelectronics, 97, 169–176. https://doi.org/10.1016/j.bios.2017.05.056.
Vigneshvar, S., Sudhakumari, C. C., Senthilkumaran, B., & Prakash, H. (2016). Recent advances in biosensor technology for potential applications: An overview. Frontiers in Bioengineering and Biotechnology, 4, 11. https://doi.org/10.3389/fbioe.2016.00011.
Liu, Y., Liu, Y., & Wang, M. (2017). Design, optimization and application of small molecule biosensor in metabolic engineering. Frontiers in Microbiology, 8, 2012. https://doi.org/10.3389/fmicb.2017.02012.
Mostafa, S., Behafarid, F., Croy, J. R., Ono, L. K., Li, L., Yang, J. C., … Cuenya, B. R. (2010). Shape-dependent catalytic properties of Pt nanoparticles. Journal of the American Chemical Society. https://doi.org/https://doi.org/10.1021/ja106679z
El-Sayed, M. A. (2004). Small is different: shape-, size-, and composition-dependent properties of some colloidal semiconductor nanocrystals. Accounts of Chemical Research, 37(5), 326–333. https://doi.org/10.1021/ar020204f.
Kelly, K. L., Coronado, E., Zhao, L. L., & Schatz, G. C. (2003). The optical properties of metal nanoparticles: The influence of size, shape, and dielectric environment. The Journal of Physical Chemistry B. https://doi.org/10.1021/jp026731y.
Jain, P. K., Lee, K. S., El-Sayed, I. H., & El-Sayed, M. A. (2006). Calculated absorption and scattering properties of gold nanoparticles of different size, shape, and composition: Applications in biological imaging and biomedicine. The Journal of Physical Chemistry B. https://doi.org/10.1021/jp057170o.
Nehl, C. L., & Hafner, J. H. (2008). Shape-dependent plasmon resonances of gold nanoparticles. Journal of Materials Chemistry. https://doi.org/10.1039/B714950F.
Kumar, S., Chauhan, D., Renugopalakrishnan, V., & Malhotra, B. D. (2020). Biofunctionalized nanodot zirconia-based efficient biosensing platform for noninvasive oral cancer detection. MRS Communications, 10(4), 652–659. https://doi.org/10.1557/mrc.2020.75.
Chan, Y. C., Chen, C. W., Chan, M. H., Chang, Y. C., Chang, W. M., Chi, L. H., … Hsiao, M. (2016). MMP2-sensing up-conversion nanoparticle for fluorescence biosensing in head and neck cancer cells. Biosensors & Bioelectronics, 80, 131–139. https://doi.org/https://doi.org/10.1016/j.bios.2016.01.049
Zhang, Y., Chen, R., Xu, L., Ning, Y., Xie, S., & Zhang, G. J. (2015). Silicon nanowire biosensor for highly sensitive and multiplexed detection of oral squamous cell carcinoma biomarkers in saliva. Analytical Sciences, 31(2), 73–78. https://doi.org/10.2116/analsci.31.73.
Zheng, Y., Liang, W., Yuan, Y., Xiong, C., Xie, S., Wang, H., … Yuan, R. (2016). Wavelength-resolved simultaneous photoelectrochemical bifunctional sensor on single interface: A newly in vitro approach for multiplexed DNA monitoring in cancer cells. Biosensors & Bioelectronics, 81, 423-430. https://doi.org/https://doi.org/10.1016/j.bios.2016.03.032
Lingen, M. W., Kalmar, J. R., Karrison, T., & Speight, P. M. (2008). Critical evaluation of diagnostic aids for the detection of oral cancer. Oral Oncology, 44(1), 10–22. https://doi.org/10.1016/j.oraloncology.2007.06.011.
Yang, H., Xiang, Y., Guo, X., Wu, Y., Wen, Y., & Yang, H. (2018). Diazo-reaction-based SERS substrates for detection of nitrite in saliva. Sensors and Actuators B: Chemical, 271, 118–121. https://doi.org/10.1016/j.snb.2018.05.111.
Ding, S., Das, S. R., Brownlee, B. J., Parate, K., Davis, T. M., Stromberg, L. R., … Claussen, J. C. (2018). CIP2A immunosensor comprised of vertically-aligned carbon nanotube interdigitated electrodes towards point-of-care oral cancer screening. Biosensors and Bioelectronics, 117, 68-74. https://doi.org/https://doi.org/10.1016/j.bios.2018.04.016
Kumar, S., Kumar, S., Tiwari, S., Srivastava, S., Srivastava, M., Yadav, B. K., … Malhotra, B. D. (2015). Biofunctionalized nanostructured zirconia for biomedical application: A smart approach for oral cancer detection. Advanced Science, 2(8), 1500048. https://doi.org/https://doi.org/10.1002/advs.201500048
Kah, J. C. Y., Kho, K. W., Lee, C. G. L., James, C., Sheppard, R., Shen, Z. X., … Olivo, M. C. (2007). Early diagnosis of oral cancer based on the surface plasmon resonance of gold nanoparticles. International Journal of Nanomedicine, 2(4), 785–798
Wang, S., Wang, H., Jiao, J., Chen, K.-J., Owens, G. E., Kamei, K., … Tseng, H.-R. (2009). Three-dimensional nanostructured substrates toward efficient capture of circulating tumor cells. Angewandte Chemie International Edition, 48(47), 8970–8973. https://doi.org/https://doi.org/10.1002/anie.200901668
Lifson, M. A., Basu Roy, D., & Miller, B. L. (2014). Enhancing the detection limit of nanoscale biosensors via topographically selective functionalization. Analytical Chemistry, 86(2), 1016–1022. https://doi.org/10.1021/ac401523e.
Liu, W.-W., Chen, Z.-L., & Jiang, X.-Y. (2009). Methods for cell micropatterning on two-dimensional surfaces and their applications in biology. Chinese Journal of Analytical Chemistry, 37(7), 943–949. https://doi.org/10.1016/S1872-2040(08)60113-9.
Whitesides, G. M., & Christopher Love, J. (2001). The art of building small. Scientific American, 285(3), 38–47. https://doi.org/10.1038/scientificamerican0901-38.
Lin, M., Chen, J.-F., Lu, Y.-T., Zhang, Y., Song, J., Hou, S., … Tseng, H.-R. (2014). Nanostructure embedded microchips for detection, isolation, and characterization of circulating tumor cells. Accounts of Chemical Research, 47(10), 2941–2950. https://doi.org/https://doi.org/10.1021/ar5001617
Choi, K. M. (2005). Photopatternable silicon elastomers with enhanced mechanical properties for high-fidelity nanoresolution soft lithography. The Journal of Physical Chemistry B. https://doi.org/10.1021/jp050302t.
Wei, F., Liao, W., Xu, Z., Yang, Y., Wong, D. T., & Ho, C.-M. (2009). Bio/abiotic interface constructed from nanoscale DNA dendrimer and conducting polymer for ultrasensitive biomolecular diagnosis. Small (Weinheim an der Bergstrasse, Germany), 5(15), 1784–1790. https://doi.org/10.1002/smll.200900369.
Aydın, E. B., Aydın, M., & Sezgintürk, M. K. (2017). A highly selective electrochemical immunosensor based on conductive carbon black and star PGMA polymer composite material for IL-8 biomarker detection in human serum and saliva. Biosensors and Bioelectronics, 117, 720–728. https://doi.org/10.1016/j.bios.2018.07.010.
Viswanathan, S., Rani, C., Vijay Anand, A., & Ho, J. A. (2009). Disposable electrochemical immunosensor for carcinoembryonic antigen using ferrocene liposomes and MWCNT screen-printed electrode. Biosensors and Bioelectronics, 24(7), 1984–1989. https://doi.org/10.1016/j.bios.2008.10.006.
Hasell, T., Lagonigro, L., Peacock, A. C., Yoda, S., Brown, P. D., Sazio, P. J. A., & Howdle, S. M. (2008). Silver nanoparticle impregnated polycarbonate substrates for surface enhanced Raman spectroscopy. Advanced Functional Materials, 18(8), 1265–1271. https://doi.org/10.1002/adfm.200701429.
Zimnitsky, D., Jiang, C., Xu, J., Lin, Z., & Tsukruk, V. V. (2007). Substrate- and time-dependent photoluminescence of quantum dots inside the ultrathin polymer LbL film. Langmuir, 23(8), 4509–4515. https://doi.org/10.1021/la0636917.
Yuan, W., Lu, Z., Wang, H., & Li, C. M. (2013). Sacrificial polymer thin-film template with tunability to construct high-density Au nanoparticle arrays and their refractive index sensing. Physical Chemistry Chemical Physics, 15(37), 15499–15507. https://doi.org/10.1039/C3CP52816B.
Jradi, K., Laour, D., Daneault, C., & Chabot, B. (2011). Control of the chemical and physical behaviour of silicon surfaces for enhancing the transition from hydrophilic to superhydrophobic surfaces. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 374(1), 33–41. https://doi.org/10.1016/j.colsurfa.2010.10.050.
Bañuls, M.-J., González-Martínez, M. Á., Sabek, J., García-Rupérez, J., & Maquieira, Á. (2019). Thiol-click photochemistry for surface functionalization applied to optical biosensing. Analytica Chimica Acta, 1060, 103–113. https://doi.org/10.1016/j.aca.2019.01.055.
Aydın, E. B., & Sezgintürk, M. K. (2018). A disposable and ultrasensitive ITO based biosensor modified by 6-phosphonohexanoic acid for electrochemical sensing of IL-1β in human serum and saliva. Analytica Chimica Acta, 1039, 41–50. https://doi.org/10.1016/j.aca.2018.07.055.
Mutin, P. H., Guerrero, G., & Vioux, A. (2003). Organic–inorganic hybrid materials based on organophosphorus coupling molecules: from metal phosphonates to surface modification of oxides. Comptes Rendus Chimie, 6(8), 1153–1164. https://doi.org/10.1016/j.crci.2003.07.006.
Silverman, B. M., Wieghaus, K. A., & Schwartz, J. (2005). Comparative properties of siloxane vs phosphonate monolayers on a key titanium alloy. Langmuir, 21(1), 225–228. https://doi.org/10.1021/la048227l.
Liu, X., Fang, C., Yan, J., Li, H., & Tu, Y. (2018). A sensitive electrochemiluminescent biosensor based on AuNP-functionalized ITO for a label-free immunoassay of C-peptide. Bioelectrochemistry, 123, 211–218. https://doi.org/10.1016/j.bioelechem.2018.05.010.
Arya, S. K., Datta, M., Singh, S. P., & Malhotra, B. D. (2007). Biosensor for total cholesterol estimation using N-(2-aminoethyl)-3-aminopropyltrimethoxysilane self-assembled monolayer. Analytical and Bioanalytical Chemistry, 389(7), 2235–2242. https://doi.org/10.1007/s00216-007-1655-7.
Song, H. Y., Zhou, X., Hobley, J., & Su, X. (2012). Comparative study of random and oriented antibody immobilization as measured by dual polarization interferometry and surface plasmon resonance spectroscopy. Langmuir, 28(1), 997–1004. https://doi.org/10.1021/la202734f.
Tajima, N., Takai, M., & Ishihara, K. (2011). Significance of antibody orientation unraveled: Well-oriented antibodies recorded high binding affinity. Analytical Chemistry, 83(6), 1969–1976. https://doi.org/10.1021/ac1026786.
Kim, E.-S., Shim, C.-K., Lee, J. W., Park, J. W., & Choi, K. Y. (2012). Synergistic effect of orientation and lateral spacing of protein G on an on-chip immunoassay. Analyst, 137(10), 2421–2430. https://doi.org/10.1039/C2AN16137K.
Chen, X., Zhou, G., Song, P., Wang, J., Gao, J., Lu, J., … Zuo, X. (2014). Ultrasensitive electrochemical detection of prostate-specific antigen by using antibodies anchored on a DNA nanostructural scaffold. Analytical chemistry, 86(15), 7337–7342. https://doi.org/https://doi.org/10.1021/ac500054x
Sanavio, B., Scaini, D., Grunwald, C., Legname, G., Scoles, G., & Casalis, L. (2010). Oriented immobilization of prion protein demonstrated via precise interfacial nanostructure measurements. ACS Nano, 4(11), 6607–6616. https://doi.org/10.1021/nn101872w.
Adam, C., Olmos, J. M., & Doneux, T. (2018). Electrochemical monitoring of the reversible folding of surface-immobilized DNA i-motifs. Langmuir, 34(9), 3112–3118. https://doi.org/10.1021/acs.langmuir.7b04088.
Malhotra, R., Patel, V., Vaque, J. P., Gutkind, J. S., & Rusling, J. F. (2010). Ultrasensitive electrochemical immunosensor for oral cancer biomarker IL-6 using carbon nanotube forest electrodes and multilabel amplification. Analytical Chemistry, 82(8), 3118–3123. https://doi.org/10.1021/ac902802b.
Tan, Y., Wei, X., Zhao, M., Qiu, B., Guo, L., Lin, Z., & Yang, H. H. (2015). Ultraselective homogeneous electrochemical biosensor for DNA species related to oral cancer based on nicking endonuclease assisted target recycling amplification. Analytical Chemistry, 87(18), 9204–9208. https://doi.org/10.1021/acs.analchem.5b01470.
Munge, B. S., Coffey, A. L., Doucette, J. M., Somba, B. K., Malhotra, R., Patel, V., … Rusling, J. F. (2011). Nanostructured immunosensor for attomolar detection of cancer biomarker interleukin-8 using massively labeled superparamagnetic particles. Angewandte Chemie International Edition, 50(34), 7915–7918. https://doi.org/https://doi.org/10.1002/anie.201102941
Mo, F., Chen, M., Meng, H., Wu, J., & Fu, Y. (2020). A DNA rolling motor for photoelectrochemical biosensing of oral cancer overexpressed 1. Sensors and Actuators B: Chemical, 309, 127824. https://doi.org/10.1016/j.snb.2020.127824.
Tiwari, S., Gupta, P. K., Bagbi, Y., Sarkar, T., & Solanki, P. R. (2017). L-cysteine capped lanthanum hydroxide nanostructures for non-invasive detection of oral cancer biomarker. Biosensors and Bioelectronics, 89(Pt 2), 1042–1052. https://doi.org/10.1016/j.bios.2016.10.020.
Kumar, S., Sharma, J. G., Maji, S., & Malhotra, B. D. (2016). A biocompatible serine functionalized nanostructured zirconia based biosensing platform for non-invasive oral cancer detection. RSC Advances, 6(80), 77037–77046. https://doi.org/10.1039/C6RA07392A.
Tuteja, S. K., Mutreja, R., Neethirajan, S., & Ingebrandt, S. (2019). Chapter 5—Bioconjugation of different nanosurfaces with biorecognition molecules for the development of selective nanosensor platforms. In A. Deep & S. Kumar (Eds.), Advances in nanosensors for biological and environmental analysis. Amsterdam: Elsevier. https://doi.org/10.1016/B978-0-12-817456-2.00005-X.
Decher, G. (1997). Fuzzy nanoassemblies: Toward layered polymeric multicomposites. Science, 277(5330), 1232. https://doi.org/10.1126/science.277.5330.1232.
Hammond, P. T. (1999). Recent explorations in electrostatic multilayer thin film assembly. Current Opinion in Colloid & Interface Science, 4(6), 430–442. https://doi.org/10.1016/S1359-0294(00)00022-4.
Xu, J. J., Zhao, W., Luo, X. L., & Chen, H. Y. (2005). A sensitive biosensor for lactate based on layer-by-layer assembling MnO2 nanoparticles and lactate oxidase on ion-sensitive field-effect transistors. Chemical Communications, 14(6), 792–794.
Franchina, J. G., Lackowski, W. M., Dermody, D. L., Crooks, R. M., Bergbreiter, D. E., Sirkar, K., … Pishko, M. V. (1999). Electrostatic immobilization of glucose oxidase in a weak acid, polyelectrolyte hyperbranched ultrathin film on gold: Fabrication, characterization, and enzymatic activity. Analytical Chemistry. https://doi.org/https://doi.org/10.1021/ac981300q
Vakurov, A., Simpson, C. E., Daly, C. L., Gibson, T. D., & Millner, P. A. (2004). Acetylcholinesterase-based biosensor electrodes for organophosphate pesticide detection: I. Modification of carbon surface for immobilization of acetylcholinesterase. Biosensors and Bioelectronics, 20(6), 1118–1125. https://doi.org/10.1016/j.bios.2004.03.039.
Goh, Y. Y., Ho, B., & Ding, J. L. (2002). A novel fluorescent protein-based biosensor for gram-negative bacteria. Applied and Environmental Microbiology, 68(12), 6343–6352.
Darder, M., Casero, E., Pariente, F., & Lorenzo, E. (2000). Biosensors based on membrane-bound enzymes immobilized in a 5-(octyldithio)-2-nitrobenzoic acid layer on gold electrodes. Analytical Chemistry, 72(16), 3784–3792. https://doi.org/10.1021/ac000276p.
Catimel, B., Scott, A. M., Lee, F. T., Hanai, N., Ritter, G., Welt, S., … Nice, E. C. (1998). Direct immobilization of gangliosides onto gold-carboxymethyldextran sensor surfaces by hydrophobic interaction: Applications to antibody characterization. Glycobiology, 8(9), 927–938. https://doi.org/https://doi.org/10.1093/glycob/8.9.927
Boozer, C., Ladd, J., Chen, S., & Jiang, S. (2006). DNA-directed protein immobilization for simultaneous detection of multiple analytes by surface plasmon resonance biosensor. Analytical Chemistry, 78(5), 1515–1519. https://doi.org/10.1021/ac051923l.
Padeste, C., Steiger, B., Grubelnik, A., & Tiefenauer, L. (2004). Molecular assembly of redox-conductive ferrocene–streptavidin conjugates—Towards bio-electrochemical devices. Biosensors and Bioelectronics, 20(3), 545–552. https://doi.org/10.1016/j.bios.2004.03.004.
Lu, Z., Li, C. M., Zhou, Q., Bao, Q.-L., & Cui, X. (2007). Covalently linked DNA/protein multilayered film for controlled DNA release. Journal of Colloid and Interface Science, 314(1), 80–88. https://doi.org/10.1016/j.jcis.2007.05.018.
Heise, A., Stamm, M., Rauscher, M., Duschner, H., & Menzel, H. (1998). Mixed silane self assembled monolayers and their in situ modification. Thin Solid Films, 327–329, 199–203. https://doi.org/10.1016/S0040-6090(98)00628-2.
Lee, J. K., Chi, Y. S., & Choi, I. S. (2004). Reactivity of acetylenyl-terminated self-assembled monolayers on gold: Triazole formation. Langmuir, 20(10), 3844–3847. https://doi.org/10.1021/la049748b.
Christiaens, P., Vermeeren, V., Wenmackers, S., Daenen, M., Haenen, K., Nesládek, M., … Wagner, P. (2006). EDC-mediated DNA attachment to nanocrystalline CVD diamond films. Biosensors and Bioelectronics, 22(2):170–177. http://doi.org/https://doi.org/10.1016/j.bios.2005.12.013
Nicolau, D. V., Taguchi, T., Taniguchi, H., & Yoshikawa, S. (1999). Protein patterning via radiation-assisted surface functionalization of conventional microlithographic materials. Colloids and Surfaces A: Physicochemical and Engineering Aspects. https://doi.org/10.1016/S0927-7757(98)00395-1.
Phizicky, E., Bastiaens, P. I. H., Zhu, H., Snyder, M., & Fields, S. (2003). Protein analysis on a proteomic scale. Nature, 422(6928), 208–215. https://doi.org/10.1038/nature01512.
Iijima, M., Yoshimoto, N., Niimi, T., Maturana, A. D., & Kuroda, S. (2013). Nanocapsule-based probe for evaluating the orientation of antibodies immobilized on a solid phase. Analyst. https://doi.org/10.1039/c3an00481c.
Choudhary, M., Yadav, P., Singh, A., Kaur, S., Ramirez-Vick, J., Chandra, P., … Singh, S. P. (2016). CD 59 targeted ultrasensitive electrochemical immunosensor for fast and noninvasive diagnosis of oral cancer. Electroanalysis, 28(10), 2565–2574. https://doi.org/https://doi.org/10.1002/elan.201600238
Jimenez Jimenez, A. M., Rodrigo, M. A. M., Milosavljevic, V., Krizkova, S., Kopel, P., Heger, Z., & Adam, V. (2017). Gold nanoparticles-modified nanomaghemite and quantum dots-based hybridization assay for detection of HPV. Sensors and Actuators B: Chemical, 240, 503–510. https://doi.org/10.1016/j.snb.2016.08.091.
Wang, Z., Fan, Y., Chen, J., Guo, Y., Wu, W., He, Y., … Fu, F. (2013). A microfluidic chip-based fluorescent biosensor for the sensitive and specific detection of label-free single-base mismatch via magnetic beads-based "sandwich" hybridization strategy. Electrophoresis, 34(15), 2177–2184. https://doi.org/https://doi.org/10.1002/elps.201300131
Song, C. K., Oh, E., Kang, M. S., Shin, B. S., Han, S. Y., Jung, M., … Lee, H. (2018). Fluorescence-based immunosensor using three-dimensional CNT network structure for sensitive and reproducible detection of oral squamous cell carcinoma biomarker. Analytica Chimica Acta 1027, 101–108. https://doi.org/https://doi.org/10.1016/j.aca.2018.04.025
Chan, Y.-C., Chan, M.-H., Chen, C.-W., Liu, R.-S., Hsiao, M., & Tsai, D. P. (2018). Near-infrared-activated fluorescence resonance energy transfer-based nanocomposite to sense MMP2-overexpressing oral cancer cells. ACS Omega, 3(2), 1627–1634. https://doi.org/10.1021/acsomega.7b01494.
Tan, G.-R., Wang, M., Hsu, C.-Y., Chen, N., & Zhang, Y. (2016). Small upconverting fluorescent nanoparticles for biosensing and bioimaging. Advanced Optical Materials, 4(7), 984–997. https://doi.org/10.1002/adom.201600141.
Nair, P. R., & Alam, M. A. (2006). Performance limits of nanobiosensors. Applied Physics Letters, 88(23), 233120. https://doi.org/10.1063/1.2211310.
Noah, N. M., & Ndangili, P. M. (2019). Current trends of nanobiosensors for point-of-care diagnostics. Journal of Analytical Methods in Chemistry, 2019, 2179718. https://doi.org/10.1155/2019/2179718.
Kumar, S., Kumar, J., & Sharma, S. N. (2018). Nanostructured titania based electrochemical impedimetric biosensor for non-invasive cancer detection. Materials Research Express, 5(12), 125405. https://doi.org/10.1088/2053-1591/aae1e2.
Kumar, S., Kumar, S., Tiwari, S., Augustine, S., Srivastava, S., Yadav, B. K., & Malhotra, B. D. (2016). Highly sensitive protein functionalized nanostructured hafnium oxide based biosensing platform for non-invasive oral cancer detection. Sensors and Actuators B: Chemical, 235, 1–10. https://doi.org/10.1016/j.snb.2016.05.047.
Kumar, S., Ashish, Kumar, S., Augustine, S., Yadav, S., Yadav, B. K., … Malhotra, B. D. (2018). Effect of Brownian motion on reduced agglomeration of nanostructured metal oxide towards development of efficient cancer biosensor. Biosensors and bioelectronics, 102, 247–255. https://doi.org/https://doi.org/10.1016/j.bios.2017.11.004
Pachauri, N., Dave, K., Dinda, A., & Solanki, P. R. (2018). Cubic CeO2 implanted reduced graphene oxide-based highly sensitive biosensor for non-invasive oral cancer biomarker detection. Journal of Materials Chemistry B, 6(19), 3000–3012. https://doi.org/10.1039/C8TB00653A.
Kumar, S., Sharma, J. G., Maji, S., & Malhotra, B. D. (2016). Nanostructured zirconia decorated reduced graphene oxide based efficient biosensing platform for non-invasive oral cancer detection. Biosensors and Bioelectronics, 78, 497–504. https://doi.org/10.1016/j.bios.2015.11.084.
Verma, S., Singh, A., Shukla, A., Kaswan, J., Arora, K., Ramirez-Vick, J., … Singh, S. P. (2017). Anti-IL8/AuNPs-rGO/ITO as an immunosensing platform for noninvasive electrochemical detection of oral cancer. ACS Applied Materials & Interfaces, 9(33), 27462–27474. https://doi.org/https://doi.org/10.1021/acsami.7b06839
Dong, T., & Pires, N. M. M. (2017). Immunodetection of salivary biomarkers by an optical microfluidic biosensor with polyethylenimine-modified polythiophene-C70 organic photodetectors. Biosensors and Bioelectronics, 94, 321–327. https://doi.org/10.1016/j.bios.2017.03.005.
Zhou, W., Ma, Y., Yang, H., Ding, Y., & Luo, X. (2011). A label-free biosensor based on silver nanoparticles array for clinical detection of serum p53 in head and neck squamous cell carcinoma. International Journal of Nanomedicine, 6, 381–386. https://doi.org/10.2147/IJN.S13249.
Yuan, L., Tu, W., Bao, J., & Dai, Z. (2015). Versatile biosensing platform for DNA detection based on a DNAzyme and restriction-endonuclease-assisted recycling. Analytical Chemistry, 87(1), 686–692. https://doi.org/10.1021/ac5034903.
Wei, F., Patel, P., Liao, W., Chaudhry, K., Zhang, L., Arellano-Garcia, M., … Wong, D. T. (2009). Electrochemical sensor for multiplex biomarkers detection. Clinical Cancer Research, 15(13), 4446–4452. https://doi.org/https://doi.org/10.1158/1078-0432.ccr-09-0050
Otieno, B. A., Krause, C. E., Latus, A., Chikkaveeraiah, B. V., Faria, R. C., & Rusling, J. F. (2014). On-line protein capture on magnetic beads for ultrasensitive microfluidic immunoassays of cancer biomarkers. Biosensors and Bioelectronics, 53, 268–274. https://doi.org/10.1016/j.bios.2013.09.054.
Torrente-Rodríguez, R. M., Campuzano, S., Ruiz-Valdepeñas Montiel, V., Gamella, M., & Pingarrón, J. M. (2016). Electrochemical bioplatforms for the simultaneous determination of interleukin (IL)-8 mRNA and IL-8 protein oral cancer biomarkers in raw saliva. Biosensors and Bioelectronics, 77, 543–548. https://doi.org/10.1016/j.bios.2015.10.016.
Epstein, J. B., Güneri, P., Boyacioglu, H., & Abt, E. (2013). The limitations of the clinical oral examination in detecting dysplastic oral lesions and oral squamous cell carcinoma. Texas Dental Journal., 130, 410–424.
Fedele, S. (2009). Diagnostic aids in the screening of oral cancer. Head & Neck Oncology, 1, 5. https://doi.org/10.1186/1758-3284-1-5.
Carreras-Torras, C., & Gay-Escoda, C. (2015). Techniques for early diagnosis of oral squamous cell carcinoma: Systematic review. Medicina Oral, Patologia Oral Y Cirugia Bucal, 20(3), e305-315. https://doi.org/10.4317/medoral.20347.
Chen, Y.-W., Lin, J.-S., Wu, C.-H., Lui, M.-T., Kao, S.-Y., & Fong, Y. (2007). Application of in vivo stain of methylene blue as a diagnostic aid in the early detection and screening of oral squamous cell carcinoma and precancer lesions. Journal of the Chinese Medical Association. https://doi.org/10.1016/S1726-4901(08)70048-0.
Du, G., Li, C., Chen, H., Chen, X., Xiao, Q., Cao, Z., … Cai, X. (2007). Rose bengal staining in detection of oral precancerous and malignant lesions with colorimetric evaluation: A pilot study. International Journal of Cancer. https://doi.org/https://doi.org/10.1002/ijc.22467
Chen, Y.-W., Lin, J.-S., Fong, J. H.-J., Wang, I.-K., Chou, S.-J., Wu, C.-H., … Kao, S.-Y. (2007). Use of methylene blue as a diagnostic aid in early detection of oral cancer and precancerous lesions. The British Journal of Oral & Maxillofacial Surgery, 45(7), 590–591. https://doi.org/https://doi.org/10.1016/j.bjoms.2006.08.017
Riaz, A., Shreedhar, B., Kamboj, M., & Natarajan, S. (2013). Methylene blue as an early diagnostic marker for oral precancer and cancer. SpringerPlus, 2(1), 95. https://doi.org/10.1186/2193-1801-2-95.
Panta, P., Rich, L. J., & Seshadri, M. (2019). Oral cancer screening: Application of vital stains as adjuncts to clinical examination. In P. Panta (Ed.), Oral cancer detection. Cham: Springer. https://doi.org/10.1007/978-3-319-61255-3_7.
Zhang, L., Bi, L., Shi, J., Zhang, Z., Cao, W., Lin, J., … Yu, Y. (2013). A quantitative diagnostic method for oral mucous precancerosis by Rose Bengal fluorescence spectroscopy. Lasers in Medical Science, 28(1), 241–246. https://doi.org/https://doi.org/10.1007/s10103-012-1054-y
Chaudhari, A., Hegde-Shetiya, S., Shirahatti, R., & Agrawal, D. (2013). Comparison of different screening methods in estimating the prevalence of precancer and cancer amongst male inmates of a jail in Maharashtra, India. Asian Pacific Journal of Cancer Prevention: APJCP, 14(2), 859–864. https://doi.org/10.7314/apjcp.2013.14.2.859.
Epstein, J. B., Scully, C., & Spinelli, J. (1992). Toluidine blue and Lugol’s iodine application in the assessment of oral malignant disease and lesions at risk of malignancy. Journal of Oral Pathology & Medicine: Official Publication of the International Association of Oral Pathologists and the American Academy of Oral Pathology, 21(4), 160–163. https://doi.org/10.1111/j.1600-0714.1992.tb00094.x.
Awan, K., Yang, Y., Morgan, P., & Warnakulasuriya, S. (2012). Utility of toluidine blue as a diagnostic adjunct in the detection of potentially malignant disorders of the oral cavity: A clinical and histological assessment. Oral Diseases, 18(8), 728–733. https://doi.org/10.1111/j.1601-0825.2012.01935.x.
Warnakulasuriya, K., & a. a. S., & Johnson, N. W. . (1996). Sensitivity and specificity of OraScan® toluidine blue mouthrinse in the detection of oral cancer and precancer. Journal of Oral Pathology & Medicine, 25(3), 97–103. https://doi.org/10.1111/j.1600-0714.1996.tb00201.x.
Patton, L. L., Epstein, J. B., & Kerr, A. R. (2008). Adjunctive techniques for oral cancer examination and lesion diagnosis: A systematic review of the literature. Centre for Reviews and Dissemination (UK). Retrieved from https://www.ncbi.nlm.nih.gov/books/NBK75331/
Rethman, M. P., Carpenter, W., Cohen, E. E. W., Epstein, J., Evans, C. A., Flaitz, C. M., … Meyer, D. M. (2010). Evidence-based clinical recommendations regarding screening for oral squamous cell carcinomas. The Journal of the American Dental Association, 141(5), 509-520. https://doi.org/https://doi.org/10.14219/jada.archive.2010.0223
Sharma, G. (2015). Diagnostic aids in detection of oral cancer: An update. World Journal of Stomatology, 4(3), 115–120.
Sambandham, T., Masthan, K. M. K., Kumar, M. S., & Jha, A. (2013). The application of vizilite in oral cancer. Journal of Clinical and Diagnostic Research: JCDR, 7(1), 185–186. https://doi.org/10.7860/JCDR/2012/5163.2704.
Ibrahim, S. S., Al-Attas, S. A., Darwish, Z. E., Amer, H. A., & Hassan, M. H. (2014). Effectiveness of the Microlux/DLTM chemiluminescence device in screening of potentially malignant and malignant oral lesions. Asian Pacific Journal of Cancer Prevention: APJCP, 15(15), 6081–6086. https://doi.org/10.7314/apjcp.2014.15.15.6081.
Rashid, A., & Warnakulasuriya, S. (2015). The use of light-based (optical) detection systems as adjuncts in the detection of oral cancer and oral potentially malignant disorders: A systematic review. Journal of Oral Pathology & Medicine: Official Publication of the International Association of Oral Pathologists and the American Academy of Oral Pathology, 44(5), 307–328. https://doi.org/10.1111/jop.12218.
Bhatia, N., Lalla, Y., Vu, A. N., & Farah, C. S. (2013). Advances in optical adjunctive AIDS for visualisation and detection of oral malignant and potentially malignant lesions. International Journal of Dentistry, 2013, 194029. https://doi.org/10.1155/2013/194029.
Liu, D., Zhao, X., Zeng, X., Dan, H., & Chen, Q. (2016). Non-invasive techniques for detection and diagnosis of oral potentially malignant disorders. The Tohoku Journal of Experimental Medicine, 238(2), 165–177. https://doi.org/10.1620/tjem.238.165.
Guze, K., Pawluk, H. C., Short, M., Zeng, H., Lorch, J., Norris, C., & Sonis, S. (2015). Pilot study: Raman spectroscopy in differentiating premalignant and malignant oral lesions from normal mucosa and benign lesions in humans. Head & Neck, 37(4), 511–517. https://doi.org/10.1002/hed.23629.
Green, B., Cobb, A. R. M., Brennan, P. A., & Hopper, C. (2014). Optical diagnostic techniques for use in lesions of the head and neck: Review of the latest developments. The British Journal of Oral & Maxillofacial Surgery, 52(8), 675–680. https://doi.org/10.1016/j.bjoms.2014.06.010.
Jerjes, W., Swinson, B., Pickard, D., Thomas, G. J., & Hopper, C. (2004). Detection of cervical intranodal metastasis in oral cancer using elastic scattering spectroscopy. Oral Oncology, 40(7), 673–678. https://doi.org/10.1016/j.oraloncology.2004.01.009.
Stephen, M. M., Jayanthi, J. L., Unni, N. G., Kolady, P. E., Beena, V. T., Jeemon, P., & Subhash, N. (2013). Diagnostic accuracy of diffuse reflectance imaging for early detection of pre-malignant and malignant changes in the oral cavity: A feasibility study. BMC Cancer, 13(1), 278. https://doi.org/10.1186/1471-2407-13-278.
Jayanthi, J. L., Nisha, G. U., Manju, S., Philip, E. K., Jeemon, P., Baiju, K. V., … Subhash, N. (2011). Diffuse reflectance spectroscopy: Diagnostic accuracy of a non-invasive screening technique for early detection of malignant changes in the oral cavity. BMJ Open, 1(1), e000071. https://doi.org/https://doi.org/10.1136/bmjopen-2011-000071
Yang, S.-W., Lee, Y.-S., Chang, L.-C., Hwang, C.-C., & Chen, T.-A. (2012). Diagnostic significance of narrow-band imaging for detecting high-grade dysplasia, carcinoma in situ, and carcinoma in oral leukoplakia. The Laryngoscope, 122(12), 2754–2761. https://doi.org/10.1002/lary.23629.
Hamdoon, Z., Jerjes, W., Upile, T., McKenzie, G., Jay, A., & Hopper, C. (2013). Optical coherence tomography in the assessment of suspicious oral lesions: An immediate ex vivo study. Photodiagnosis and Photodynamic Therapy, 10(1), 17–27. https://doi.org/10.1016/j.pdpdt.2012.07.005.
Nathan, C.-A. O., Kaskas, N. M., Ma, X., Chaudhery, S., Lian, T., Moore-Medlin, T., … Mehta, V. (2014). Confocal laser endomicroscopy in the detection of head and neck precancerous lesions. Otolaryngology-Head and Neck Surgery: Official Journal of American Academy of Otolaryngology-Head and Neck Surgery, 151(1), 73–80. https://doi.org/https://doi.org/10.1177/0194599814528660
Olivo, M., Bhuvaneswari, R., & Keogh, I. (2011). Advances in bio-optical imaging for the diagnosis of early oral cancer. Pharmaceutics, 3(3), 354–378. https://doi.org/10.3390/pharmaceutics3030354.
Spizzo, G., Fong, D., Wurm, M., Ensinger, C., Obrist, P., Hofer, C., … Went, P. (2011). EpCAM expression in primary tumour tissues and metastases: An immunohistochemical analysis. Journal of Clinical Pathology, 64(5), 415. https://doi.org/https://doi.org/10.1136/jcp.2011.090274
Coletta, R. D., Cotrim, P., Vargas, P. A., Villalba, H., Pires, F. R., de Moraes, M., & de Almeida, O. P. (2001). Basaloid squamous carcinoma of the oral cavity: Report of 2 cases and study of AgNOR, PCNA, p53, and MMP expression. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology, and Endodontology, 91(5), 563–569. https://doi.org/10.1067/moe.2001.113161.
Mascolo, M., Siano, M., Ilardi, G., Russo, D., Merolla, F., Rosa, G. D., & Staibano, S. (2012). Epigenetic disregulation in oral cancer. International Journal of Molecular Sciences, 13(2), 2331–2353.
Pintos, J., Black, M. J., Sadeghi, N., Ghadirian, P., Zeitouni, A. G., Viscidi, R. P., … Franco, E. L. (2008). Human papillomavirus infection and oral cancer: A case-control study in Montreal, Canada. Oral Oncology 44(3), 242–250. https://doi.org/https://doi.org/10.1016/j.oraloncology.2007.02.005
Chikui, T., Kitamoto, E., Kawano, S., Sugiura, T., Obara, M., Simonetti, A. W., … Yoshiura, K. (2012). Pharmacokinetic analysis based on dynamic contrast-enhanced MRI for evaluating tumor response to preoperative therapy for oral cancer. Journal of Magnetic Resonance Imaging, 36(3), 589–597. https://doi.org/https://doi.org/10.1002/jmri.23704
Schöder, H., Carlson, D. L., Kraus, D. H., Stambuk, H. E., Gönen, M., Erdi, Y. E., … Wong, R. J. (2006). 18F-FDG PET/CT for detecting nodal metastases in patients with oral cancer staged N0 by clinical examination and CT/MRI. Journal of Nuclear Medicine: Official Publication, Society of Nuclear Medicine, 47(5), 755–762
Rhodus, N. L., Ho, V., Miller, C. S., Myers, S., & Ondrey, F. (2005). NF-κB dependent cytokine levels in saliva of patients with oral preneoplastic lesions and oral squamous cell carcinoma. Cancer Detection and Prevention, 29(1), 42–45. https://doi.org/10.1016/j.cdp.2004.10.003.
Brailo, V., Vucicevic-Boras, V., Lukac, J., Biocina-Lukenda, D., Zilic-Alajbeg, I., Milenovic, A., & Balija, M. (2012). Salivary and serum interleukin 1 beta, interleukin 6 and tumor necrosis factor alpha in patients with leukoplakia and oral cancer. Medicina oral, patología oral y cirugía bucal, 17(1), e10.
Katakura, A., Kamiyama, I., Takano, N., Shibahara, T., Muramatsu, T., Ishihara, K., … Shouno, T. (2007). Comparison of salivary cytokine levels in oral cancer patients and healthy subjects. The Bulletin of Tokyo Dental College, 48(4), 199–203
Al-Mahfoud, M. M., Al-Saimary, I. E., & Al-Shawi, A. A. (2019). The expression of Interlukin 2(IL-2), Interlukin 8(IL-8) and Interlukin 6(IL-6) in patients with oral and oropharyngeal squamous cell carcinoma in Basrah city (A case control study). Journal of Physics: Conference Series, 1294, 062003. https://doi.org/10.1088/1742-6596/1294/6/062003.
Pickering, V., Jordan, R. C. K., & Schmidt, B. L. (2007). Elevated salivary endothelin levels in oral cancer patients—A pilot study. Oral Oncology, 43(1), 37–41. https://doi.org/10.1016/j.oraloncology.2005.12.027.
Irfan, S., Tandon, N., Srivastava, A., Sharma, R., & Kumar, V. (2015). Serum endothelin-1 as biomarker in oral cancer: A case control study International. Journal of Bioassays. https://doi.org/10.21746/IJBIO.2015.11.
Rajkumar, K., Ramya, R., Nandhini, G., Rajashree, P., Ramesh Kumar, A., & Nirmala Anandan, S. (2015). Salivary and serum level of CYFRA 21–1 in oral precancer and oral squamous cell carcinoma. Oral Diseases, 21(1), 90–96. https://doi.org/10.1111/odi.12216.
Li, T., & Yang, M. (2011). Electrochemical sensor utilizing ferrocene loaded porous polyelectrolyte nanoparticles as label for the detection of protein biomarker IL-6. Sensors and Actuators B: Chemical, 158(1), 361–365. https://doi.org/10.1016/j.snb.2011.06.035.
Liu, D., Zhao, X., Zeng, X., Dan, H., & Chen, Q. (2016). Paper-based plasmonic platform for sensitive, noninvasive, and rapid cancer screening. Biosensors and Bioelectronics, 54, 128–134. https://doi.org/10.1016/j.bios.2013.10.067.
Wang, Y., Wang, Y., Pang, X., Du, B., Li, H., Wu, D., & Wei, Q. (2015). Ultrasensitive sandwich-type electrochemical immunosensor based on dual signal amplification strategy using multifunctional graphene nanocomposites as labels for quantitative detection of tissue polypeptide antigen. Sensors and Actuators B: Chemical, 214, 124–131. https://doi.org/10.1016/j.snb.2015.03.021.
Wang, H.-C., Nguyen, N.-V., Lin, R.-Y., & Jen, C.-P. (2017). Characterizing esophageal cancerous cells at different stages using the dielectrophoretic impedance measurement method in a microchip. Sensors. https://doi.org/10.3390/s17051053.
Wu, I. C., Weng, Y.-H., Lu, M.-Y., Jen, C.-P., Fedorov, V. E., Chen, W. C., … Wang, H.-C. (2017). Nano-structure ZnO/Cu2O photoelectrochemical and self-powered biosensor for esophageal cancer cell detection. Optics Express. https:doi.org/https://doi.org/10.1364/OE.25.007689
Kumar, S., Panwar, S., Kumar, S., Augustine, S., & Malhotra, B. D. (2019). Biofunctionalized nanostructured yttria modified non-invasive impedometric biosensor for efficient detection of oral cancer. Nanomaterials (Basel, Switzerland). https://doi.org/10.3390/nano9091190.
Verma, S., & Singh, S. P. (2019). Non-invasive oral cancer detection from saliva using zinc oxide–reduced graphene oxide nanocomposite based bioelectrode. MRS Communications, 9(4), 1227–1234. https://doi.org/10.1557/mrc.2019.138.
Chen, J., Zhang, J., Guo, Y., Li, J., Fu, F., Yang, H. H., & Chen, G. (2011). An ultrasensitive electrochemical biosensor for detection of DNA species related to oral cancer based on nuclease-assisted target recycling and amplification of DNAzyme. Chemical Communications, 47(28), 8004–8006. https://doi.org/10.1039/c1cc11929j.
Wang, J. H., Wang, B., Liu, Q., Li, Q., Huang, H., Song, L., … Chu, P. K. (2013). Bimodal optical diagnostics of oral cancer based on Rose Bengal conjugated gold nanorod platform. Biomaterials, 34(17), 4274–4283. https://doi.org/https://doi.org/10.1016/j.biomaterials.2013.02.012
Islam, M. N., Masud, M. K., Nguyen, N.-T., Gopalan, V., Alamri, H. R., Alothman, Z. A., … Shiddiky, M. J. A. (2018). Gold-loaded nanoporous ferric oxide nanocubes for electrocatalytic detection of microRNA at attomolar level. Biosensors and Bioelectronics, 101, 275-281. https://doi.org/https://doi.org/10.1016/j.bios.2017.09.027
Acknowledgements
The authors would like to acknowledge the Department of Health Research – Multidisciplinary Research Unit, King George’s Research Unit, Lucknow for providing the required infrastructure to prepare this manuscript. SV would like to acknowledge the Indian Council of Medical Research, New Delhi, India for the Research Associate fellowship.
Funding
No funding was received to assist with the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
JS and SK conceived the idea, JS and SV prepared the manuscript, JS performed the literature search and data analysis, JS and SV prepared illustration, SK and DM helped in drafting and critical review of the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that there are no conflicts of interest associated with this manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Singhal, J., Verma, S., Kumar, S. et al. Recent Advances in Nano-Bio-Sensing Fabrication Technology for the Detection of Oral Cancer. Mol Biotechnol 63, 339–362 (2021). https://doi.org/10.1007/s12033-021-00306-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12033-021-00306-x