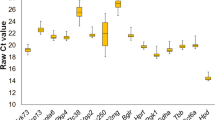
Abstract
With the epidemic proportions of obesity worldwide and the concurrent prevalence of hepatic steatosis, there is an urgent need for better understanding the intrinsic mechanism of hepatic steatosis, especially the changes of gene expression underlying the development of hepatic steatosis and its associated abnormal liver function. Quantitative real-time PCR (qRT-PCR) is a sensitive and highly reproducible technique of gene expression analysis. However, for accurate and reliable gene expression results, it is vital to have an internal control gene expressed at constant levels under all the experimental conditions being analyzed for. In this study, the authors validated candidate reference genes suitable for qRT-PCR profiling experiments using livers from control mice and high fat diet-induced obese mice. Cross-validation of expression stability of ten selected reference genes using three popular algorithms, GeNorm, NormFinder, and BestKeeper found HPRT1 and GAPDH as most stable reference genes. Thus, HPRT1 and GAPDH are recommended as stable reference genes most suitable for gene expression studies in the development of hepatic steatosis.




Similar content being viewed by others
References
Adams, L. A., & Lindor, K. D. (2007). Nonalcoholic fatty liver disease. Annals of Epidemiology, 17, 863–869.
Leclercq, L. A., Da Silva Morais, A., Schroyen, B., et al. (2007). Insulin resistance in hepatocytes and sinusoidal liver cells: Mechanisms and consequences. Journal of Hepatology, 47, 142–145.
Guo, F., & Cavener, D. R. (2007). The GCN2 eIF2alpha kinase regulates fatty-acid homeostasis in the liver during deprivation of an essential amino acid. Cell Metabolism, 5, 103–114.
Brookes, M. J., & Cooper, B. T. (2007). Hypertension and fatty liver: Guilty by association? Journal of Human Hypertension, 21, 264–270.
Saadeh, S. (2007). Nonalcoholic fatty liver disease and obesity. Nutrition in Clinical Practice, 22, 1–10.
Byrne, C. D. (2009). Hypoxia and non-alcoholic fatty liver disease. Clinical Science (London), 118, 397–400.
De Alwis, N. M., & Day, C. P. (2008). Non-alcoholic fatty liver disease: The mist gradually clears. Journal of Hepatology, 48, S104–S112.
Farrell, G. C., & Larter, C. Z. (2006). Nonalcoholic fatty liver disease: From steatosis to cirrhosis. Hepatology, 43, 99–112.
Younossi, Z. M., Diehl, A. M., & Ong, J. P. (2002). Nonalcoholic fatty liver disease: An agenda for clinical research. Hepatology, 35, 746–752.
Hanley, A. J., Williams, K., Festa, A., et al. (2005). Liver markers and development of the metabolic syndrome. Diabetes, 54, 3140–3147.
De Jonge, H. J., Fehrmann, R. S., De Bont, E. S., et al. (2007). Evidence based selection of housekeeping genes. PloS ONE, 2, e898.
Cikos, S., & Koppel, J. (2009). Transformation of real-time PCR fluorescence data to target gene quantity. Analytical Biochemistry, 384, 1–10.
Bustin, S. A. (2002). Quantification of mRNA using real-time reverse transcription PCR (RT-PCR): Trends and problems. Journal of Molecular Endocrinology, 29, 23–39.
Huggett, J., Dheda, K., Bustin, S., et al. (2005). Strategies and considerations. Genes and Immunity, 6, 279–284.
Vandesompele, J., De Preter, K., Pattyn, F., et al. (2002). Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biology, 3, 0034.1–0034.11.
Radonić, A., Thulke, S., Mackay, I. M., et al. (2004). Guideline to reference gene selection for quantitative real-time PCR. Biochemical and Biophysical Research Communications, 313, 856–862.
Andersen, C. L., Jensen, J. L., & Orntoft, T. F. (2004). Normalization of real-time quantitative reverse transcription-PCR data: A model-based variance estimation approach to identify genes suited for normalization applied to bladder and colon cancer data sets. Cancer Research, 64, 5245–5250.
Pfaffl, M. W., Tichopad, A., Prgomet, C., et al. (2004). Determination of stable housekeeping genes, differentially regulated target genes, and sample integrity: BestKeeper—Excel-based tool using pair-wise correlations. Biotechnology Letters, 26, 509–515.
Yang, L., Zhang, Y., Wang, L., et al. (2010). Amelioration of high fat diet induced liver lipogenesis and hepatic steatosis by interleukin-22. Journal of Hepatology, 53, 339–347.
Mehta, R., Birerdinc, A., Hossain, N., et al. (2010). Validation of endogenous reference genes for qRT-PCR analysis of human visceral adipose samples. BMC Molecular Biology, 11, 39–49.
Shoelson, S. E., Lee, J., & Goldfine, A. B. (2006). Inflammation and insulin resistance. Journal of Clinical Investigation, 116, 1793–1801.
Carter-Kent, C., Zein, N. N., & Feldstein, A. E. (2008). Cytokines in the pathogenesis of fatty liver and disease progression to steatohepatitis: Implications for treatment. American Journal of Gastroenterology, 103, 1036–1042.
Gantner, F., Leist, M., Lohse, A. W., et al. (1995). Concanavalin A-induced T-cell-mediated hepatic injury in mice. The role of tumor necrosis factor. Hepatology, 21, 190–198.
Fausto, N. (2006). Involvement of the innate immune system in liver regeneration and injury. Journal of Hepatology, 45, 347–349.
Feng, D., Wang, Y., Mei, Y., et al. (2009). Stearoyl-CoA desaturase 1 deficiency protects mice from immune-mediated liver injury. Laboratory Investigation, 89, 222–230.
Shi, G., Zhang, Z., Feng, D., et al. (2010). Selection of reference genes for quantitative real-time reverse transcription-polymerase chain reaction in concanavalin A-induced hepatitis model. Analytical Biochemistry, 401, 81–90.
Halder, R. C., Aguilera, C., Maricic, I., et al. (2007). Type II NKT cell-mediated anergy induction in type I NKT cells prevents inflammatory liver disease. Journal of Clinical Investigation, 117, 2302–2312.
Beldi, G., Wu, Y., Banz, Y., et al. (2008). Natural killer T cell dysfunction in CD39-null mice protects against concanavalin A-induced hepatitis. Hepatology, 48, 841–852.
Nakashima, H., Kinoshita, M., Nakashima, M., et al. (2008). Superoxide produced by Kupffer cells is an essential effector in concanavalin A-induced hepatitis in mice. Hepatology, 48, 1979–1988.
Acknowledgments
This study was supported by the grants from 973 Project (no. 2006CB503904), 863 Project (no. 2006 AA 02A409), the National Natural Science Foundation of China (no. 30725037, 30971077, and 30971385), and Shanghai Committee for Science and Technology (no. 07JC14042). The authors thank Jiulan Cui of Shanghai Jiao-Tong University School of Medicine for maintaining the mouse colony.
Author information
Authors and Affiliations
Corresponding author
Additional information
Lingyan Xu and Xinran Ma contributed equally to this study.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.

12033_2010_9366_MOESM1_ESM.jpg
Supplementary Fig. 1. Verification of amplified products and sequencing reactions. (A) PCR products were checked on 1.5% agarose gel and shown to have the expected size. (B) Representative images of sequences of amplification products by direct sequencing with our designed primers. (JPEG 791 kb)
Rights and permissions
About this article
Cite this article
Xu, L., Ma, X., Cui, B. et al. Selection of Reference Genes for qRT-PCR in High Fat Diet-Induced Hepatic Steatosis Mice Model. Mol Biotechnol 48, 255–262 (2011). https://doi.org/10.1007/s12033-010-9366-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12033-010-9366-2