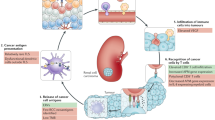
Abstract
Efforts in cancer immunotherapy aim to counteract evasion mechanisms and stimulate the immune system to recognise and attack cancer cells effectively. Combination therapies that target multiple aspects of immune evasion are being investigated to enhance the overall efficacy of cancer immunotherapy. PD-1 (Programmed Cell Death Protein 1), CTLA-4 (Cytotoxic T-Lymphocyte Antigen 4), LAG-3 (Lymphocyte-Activation Gene 3), and TIM-3 (T Cell Immunoglobulin and Mucin Domain-Containing Protein3) are all immune checkpoint receptors that play crucial roles in regulating the immune response and maintaining self-tolerance often exploited by cancer cells to evade immune surveillance. Antibodies targeted against immune checkpoint inhibitors such as anti-PD-1 antibodies (e.g., pembrolizumab, nivolumab), anti-CTLA-4 antibodies (e.g., Ipilimumab), and experimental drugs targeting LAG-3 and TIM-3, aim to block these interactions and unleash the immune system’s ability to recognise and destroy cancer cells. The US FDA has approved different categories of immune checkpoint inhibitors that have been utilised successfully in some patients with metastatic melanoma, renal cell carcinoma, head and neck cancers, and non-small lung cancer. Although several immune checkpoint inhibitor antibodies have been developed, they exhibited immune-related adverse effects, resulting in hypophysitis, diabetes, and neurological issues. These adverse effects of antibodies can be reduced by developing aptamer against the target. Aptamers offer several advantages over traditional antibodies, such as improved specificity, reduced immunogenicity, and flexible design for reduced adverse effects that specifically target and block protein–protein or receptor-ligand interactions involved in immune checkpoint pathways. The current study aims to review the function of particular immune checkpoint inhibitors along with developed aptamer-mediated antitumor cytotoxicity in cancer treatment.


Similar content being viewed by others
Abbreviations
- PD-1:
-
Programmed cell death 1
- CTLA-4:
-
Cytotoxic T lymphocyte-associated protein 4
- LAG-3:
-
Lymphocyte-Activation Gene 3
- PD-L1:
-
Programmed cell death ligand1
- PD-L2:
-
Programmed cell death ligand2
- TIM-3:
-
T cell immunoglobulin and mucin-domain containing-3
- FDA:
-
Food and Drug Administration
- IARC:
-
International Agency for Research on Cancer
- ICIs:
-
Immune checkpoint Inhibitors
- NSCLC:
-
Non-small cell lung cancer
- VISTA:
-
V-domain Ig suppressor of T cell activation
- BTLA:
-
B and T cell lymphocyte attenuator
- TIGIT:
-
T cell immunoglobulin and ITIM domain
- B7-H3:
-
B7 homolog 3 protein
- APCs:
-
Antigen Presenting Cells
- TME:
-
Tumour Microenvironment
- TCR:
-
T-cell receptor
- HAVCR2:
-
Hepatitis A virus cellular receptor 2
- mAbs:
-
Monoclonal Antibodies
- IbAEs:
-
Immune-based adverse events
- SELEX:
-
Systemic Evolution of Ligands by Exponential Enrichment
- VEGF:
-
Vascular Endothelial Growth Factor
- IDO:
-
Indoleamine 2,3-dioxygenase
- DCs:
-
Dendritic cells
- NKCs:
-
Natural Killer Cells
- MHC II:
-
Major Histocompatibility Complex II
References
Aamdal E, Inderberg EM, Ellingsen EB, Rasch W, Brunsvig PF, Aamdal S, Heintz KM, Vodák D, Nakken S, Hovig E, Nyakas M. Combining a universal telomerase based cancer vaccine with Ipilimumab in patients with metastatic melanoma-five-year follow up of a phase I/IIa trial. Front Immunol. 2021;12: 663865.
Acharya N, Sabatos-Peyton C, Anderson AC. Tim-3 finds its place in the cancer immunotherapy landscape. J Immunother Cancer. 2020;8(1): e000911.
Andrews LP, Marciscano AE, Drake CG, Vignali DA. LAG 3 (CD 223) as a cancer immunotherapy target. Immunol Rev. 2017;276(1):80–96.
Attia P, Phan GQ, Maker AV, Robinson MR, Quezado MM, Yang JC, Sherry RM, Topalian SL, Kammula US, Royal RE, Restifo NP. Autoimmunity correlates with tumor regression in patients with metastatic melanoma treated with anti–cytotoxic T-lymphocyte antigen-4. J Clin Oncol. 2005;23(25):6043.
Avery L, Filderman J, Szymczak-Workman AL, Kane LP. Tim-3 co-stimulation promotes short-lived effector T cells, restricts memory precursors, and is dispensable for T cell exhaustion. Proc Natl Acad Sci. 2018;115(10):2455–60.
Bagchi S, Yuan R, Engleman EG. Immune checkpoint inhibitors for the treatment of cancer: clinical impact and mechanisms of response and resistance. Annu Rev Pathol. 2021;16:223–49.
Baixeras E, Huard B, Miossec CA, Jitsukawa S, Martin M, Hercend T, Auffray C, Triebel F, Piatier-Tonneau D. Characterization of the lymphocyte activation gene 3-encoded protein. A new ligand for human leukocyte antigen class II antigens. J Exp Med. 1992;176(2):327–37.
Bardhan K, Anagnostou T, Boussiotis VA. The PD1: PD-L1/2 pathway from discovery to clinical implementation. Front Immunol. 2016;7:550.
Buff MC, Schäfer F, Wulffen B, Müller J, Pötzsch B, Heckel A, Mayer G. Dependence of aptamer activity on opposed terminal extensions: improvement of light-regulation efficiency. Nucleic Acids Res. 2010;38(6):2111–8.
Camacho LH, Antonia S, Sosman J, Kirkwood JM, Gajewski TF, Redman B, Pavlov D, Bulanhagui C, Bozon VA, Gomez-Navarro J, Ribas A. Phase I/II trial of tremelimumab in patients with metastatic melanoma. J Clin Oncol. 2009;27(7):1075–81.
Carthon BC, Wolchok JD, Yuan J, Kamat A, Ng Tang DS, Sun J, Ku G, Troncoso P, Logothetis CJ, Allison JP, Sharma P. Preoperative CTLA-4 blockade: tolerability and immune monitoring in the setting of a presurgical clinical trialpreoperative CTLA-4 blockade. Clin Cancer Res. 2010;16(10):2861–71.
Chen G, Huang AC, Zhang W, Zhang G, Wu M, Xu W, Yu Z, Yang J, Wang B, Sun H, Xia H. Exosomal PD-L1 contributes to immunosuppression and is associated with anti-PD-1 response. Nature. 2018;560(7718):382–6.
Chen W, Jin W, Wahl SM. Engagement of cytotoxic T lymphocyte–associated antigen 4 (CTLA-4) induces transforming growth factor β (TGF-β) production by murine CD4+ T cells. J Exp Med. 1998;188(10):1849–57.
Cohen MG, Purdy DA, Rossi JS, Grinfeld LR, Myles SK, Aberle LH, Greenbaum AB, Fry E, Chan MY, Tonkens RM, Zelenkofske S. First clinical application of an actively reversible direct factor IXa inhibitor as an anticoagulation strategy in patients undergoing percutaneous coronary intervention. Circulation. 2010;122(6):614–22.
Comin-Anduix B, Lee Y, Jalil J, Algazi A, de la Rocha P, Camacho LH, Bozon VA, Bulanhagui CA, Seja E, Villanueva A, Straatsma BR. Detailed analysis of immunologic effects of the cytotoxic T lymphocyte-associated antigen 4-blocking monoclonal antibody tremelimumab in peripheral blood of patients with melanoma. J Transl Med. 2008;6(1):1–4.
Comprehensive Cancer Information National Cancer Institute. [Online]. Available: https://www.cancer.gov/.
Cooper WA, Tran T, Vilain RE, Madore J, Selinger CI, Kohonen-Corish M, Yip P, Yu B, O’Toole SA, McCaughan BC, Yearley JH. PD-L1 expression is a favorable prognostic factor in early stage non-small cell carcinoma. Lung Cancer. 2015;89(2):181–8.
Curiel TJ, Wei S, Dong H, Alvarez X, Cheng P, Mottram P, Krzysiek R, Knutson KL, Daniel B, Zimmermann MC, David O. Blockade of B7–H1 improves myeloid dendriticcell–mediated antitumor immunity. Nat Med. 2003;9(5):562–7.
Dariavach P, Mattéi MG, Golstein P, Lefranc MP. Human Ig superfamily CTLA-4 gene: chromosomal localizsation and identity of protein sequence between murine and human CTLA-4 cytoplasmic domains. Eur J Immunol. 1988;18(12):1901–5.
Desai J, Deva S, Lee JS, Lin CC, Yen CJ, Chao Y, Keam B, Jameson M, Hou MM, Kang YK, Markman B. Phase IA/IB study of single-agent tislelizumab, an investigational anti-PD-1 antibody, in solid tumors. J Immunother Cancer. 2020;8(1): e000453.
Dunn GP, Bruce AT, Ikeda H, Old LJ, Schreiber RD. Cancer immunoediting: from immunosurveillance to tumor escape. Nat Immunol. 2002;3(11):991–8.
Ellington AD, Szostak JW. In vitro selection of RNA molecules that bind specific ligands. Nature. 1990;346(6287):818–22.
Fehrenbacher L, Spira A, Ballinger M, Kowanetz M, Vansteenkiste J, Mazieres J, Park K, Smith D, Artal-Cortes A, Lewanski C, Braiteh F. Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): a multicentre, open-label, phase 2 randomised controlled trial. The Lancet. 2016;387(10030):1837–46.
Freeman GJ, Casasnovas JM, Umetsu DT, DeKruyff RH. TIM genes: a family of cell surface phosphatidylserine receptors that regulate innate and adaptive immunity. Immunol Rev. 2010;235(1):172–89.
Freeman GJ, Long AJ, Iwai Y, Bourque K, Chernova T, Nishimura H, Fitz LJ, Malenkovich N, Okazaki T, Byrne MC, Horton HF. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med. 2000;192(7):1027–34.
Fu Z, Xiang J. Aptamers, the nucleic acid antibodies, in cancer therapy. Int J Mol Sci. 2020;21(8):2793.
Gao T, Mao Z, Li W, Pei R. Anti-PD-L1 DNA aptamer santagonises the interaction of PD-1/PD-L1 with antitumor effect. J Mater Chem B. 2021;9(3):746–56.
Gaynor Nicola, Crown John, Collins Denis M. Immune checkpoint inhibitors: key trials and an emerging role in breast cancer. Semin Cancer Biol. 2022;79:44–57.
Gefen T, Castro I, Muharemagic D, Puplampu-Dove Y, Patel S, Gilboa E. A TIM-3 oligonucleotide aptamer enhances T cell functions and potentiates tumor immunity in mice. Mol Ther. 2017;25(10):2280–8.
Gibney GT, Kudchadkar RR, DeConti RC, Thebeau MS, Czupryn MP, Tetteh L, Eysmans C, Richards A, Schell MJ, Fisher KJ, Horak CE. Safety, correlative markers, and clinical results of adjuvant nivolumab in combination with vaccine in resected high-risk metastatic melanomaadjuvant anti–PD-1 therapy in resected melanoma patients. Clin Cancer Res. 2015;21(4):712–20.
Gibney GT, Kudchadkar RR, DeConti RC, Thebeau MS, Czupryn MP, Tetteh L, Eysmans C, Richards A, Schell MJ, Fisher KJ, Horak CE. Safety, correlative markers, and clinical results of adjuvant nivolumab in combination with vaccine in resected high-risk metastatic melanoma adjuvant anti–PD-1 therapy in resected melanoma patients. Clin Cancer Res. 2015;21(4):712–20.
Grasselly C, Denis M, Bourguignon A, Talhi N, Mathe D, Tourette A, Serre L, Jordheim LP, Matera EL, Dumontet C. The antitumor activity of combinations of cytotoxic chemotherapy and immune checkpoint inhibitors is model-dependent. Front Immunol. 2018;9:2100.
Gross JA, St John T, Allison JP. The murine homologue of the T lymphocyte antigen CD28. Molecular cloning and cell surface expression. J Immunol. 1990;144(8):3201–10.
Guo P. The emerging field of RNA nanotechnology. Nat Nanotechnol. 2010;5(12):833–42.
Harrington K, Kong A, Mach N, Rordorf T, Corral J, Espeli V, Treichel S, Cheng J, Kim JJ, Chesney J. Early safety from phase 1b/3, multicenter, open-label, srandomised trial of talimogene laherparepvec (T-VEC)+ pembrolizumab (pembro) for recurrent or metastatic squamous cell carcinoma of the head and neck (R/M SCCHN): MASTERKEY-232. Ann Oncol. 2017;28: v394.
Harrington Kevin J, Kong Anthony Hee, Mach Nicolas, Rordorf Tamara, Jaime Jesus Corral, Espeli Vittoria, Treichel Sheryl, Gumuscu Burak, Kim Jenny J, Chesney Jason Alan. Safety and preliminary efficacy of talimogene laherparepvec (T-VEC) in combination (combo) with pembrobrolizumab (Pembro) in patients (pts) with recurrent or metastatic squamous cell carcinoma of the head and neck (R/M HNSCC): a multicenter, phase 1b study (MASTERKEY-232). J Clin Oncol. 2018;36:6036–6036.
He F, Wen N, Xiao D, Yan J, Xiong H, Cai S, Liu Z, Liu Y. Aptamer-based targeted drug delivery systems: current potential and challenges. Curr Med Chem. 2020;27(13):2189–219.
He J, Hu Y, Hu M, Li B. Development of PD-1/PD-L1 pathway in tumor immune microenvironment and treatment for non-small cell lung cancer. Sci Rep. 2015;5(1):1–9.
He Y, Cao J, Zhao C, Li X, Zhou C, Hirsch FR. TIM-3 a promising target for cancer immunotherapy. OncoTargets Ther. 2018;11:7005–9.
He Y, Rivard CJ, Rozeboom L, Yu H, Ellison K, Kowalewski A, Zhou C, Hirsch FR. Lymphocyte-activation gene-3, an important immune checkpoint in cancer. Cancer Sci. 2016;107(9):1193–7.
Healy JM, Lewis SD, Kurz M, Boomer RM, Thompson KM, Wilson C, McCauley TG. Pharmacokinetics and biodistribution of novel aptamer compositions. Pharm Res. 2004;21:2234–46.
Heo K, Min SW, Sung HJ, Kim HG, Kim HJ, Kim YH, Choi BK, Han S, Chung S, Lee ES, Chung J. An aptamer-antibody complex (oligobody) as a novel delivery platform for targeted cancer therapies. J Control Release. 2016;229:1–9.
Herrmann A, Priceman SJ, Kujawski M, Xin H, Cherryholmes GA, Zhang W, Zhang C, Lahtz C, Kowolik C, Forman SJ, Kortylewski M. CTLA4 aptamer delivers STAT3 siRNA to tumor-associated and malignant T cells. J Clin Investig. 2014;124(7):2977–87.
Hervas-Stubbs S, Soldevilla MM, Villanueva H, Mancheño U, Bendandi M, Pastor F. Identification of TIM3 2′-fluoro oligonucleotide aptamer by HT-SELEX for cancer immunotherapy. Oncotarget. 2016;7(4):4522.
Hodi FS, Mihm MC, Soiffer RJ, Haluska FG, Butler M, Seiden MV, Davis T, Henry-Spires R, MacRae S, Willman A, Padera R. Biologic activity of cytotoxic T lymphocyte-associated antigen 4 antibody blockade in previously vaccinated metastatic melanoma and ovarian carcinoma patients. Proc Natl Acad Sci. 2003;100(8):4712–7.
Hodi FS, O’day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC, Akerley W. Improved survival with Ipilimumab in patients with metastatic melanoma. New Engl J Med. 2010;363(8):711–23.
Hsueh EC, Essner R, Foshag LJ, Ollila DW, Gammon G, O’Day SJ, Boasberg PD, Stern SL, Ye X, Morton DL. Prolonged survival after complete resection of disseminated melanoma and active immunotherapy with a therapeutic cancer vaccine. J Clin Oncol. 2002;20(23):4549–54.
Hu X, Zeng H, Peng Y, Deng M, Xiang W, Liu B, Liu J, Fu Y, Hu Z, Hou W, Liu X. Targeting PD-L1 with DNA aptamers and conjugated with gemcitabine as a novel therapeutic strategy for bladder cancer chemotherapy combined with immunotherapy. Small Sci. 2023. https://doi.org/10.1002/smsc.202300104.
Huang BT, Lai WY, Chang YC, Wang JW, Yeh SD, Lin EP, Yang PC. A CTLA-4 santagonising DNA aptamer with antitumor effect. Mol Ther Nucleic Acids. 2017;8:520–8.
Huard B, Prigent P, Tournier M, Bruniquel D, Triebel F. CD4/major histocompatibility complex class II interaction analysed with CD4-and lymphocyte activation gene-3 (LAG-3)-Ig fusion proteins. Eur J Immunol. 1995;25(9):2718–21.
International Agency for Research on Cancer. IARC Biennial Report 2020–2021. Lyon: International Agency for Research on Cancer. 2021
Jaffe GJ, Westby K, Csaky KG, Monés J, Pearlman JA, Patel SS, Joondeph BC, Randolph J, Masonson H, Rezaei KA. C5 inhibitor avacincaptad pegol for geographic atrophy due to age-related macular degeneration: a srandomised pivotal phase 2/3 trial. Ophthalmology. 2021;128(4):576–86.
Johnson DB, Balko JM, Compton ML, Chalkias S, Gorham J, Xu Y, Hicks M, Puzanov I, Alexander MR, Bloomer TL, Becker JR. Fulminant myocarditis with combination immune checkpoint blockade. N Engl J Med. 2016;375(18):1749–55.
Kakavand H, Vilain RE, Wilmott JS, Burke H, Yearley JH, Thompson JF, Hersey P, Long GV, Scolyer RA. Tumor PD-L1 expression, immune cell correlates and PD-1+ lymphocytes in sentinel lymph node melanoma metastases. Mod Pathol. 2015;28(12):1535–44.
Kamat A, Tang DN. CTLA-4 blockade increases IFNgamma-producing CD4+ ICOSh’cells to shift the ratio of effector to regulatory T cells in cancer patients. Proc Natl Acad Sci USA. 2008;105(39):14987–92.
Kang JH, Bluestone JA, Young A. Predicting and preventing immune checkpoint inhibitor toxicity: targeting cytokines. Trends Immunol. 2021;42(4):293–311.
Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008;26:677–704.
Kitchlu A, Fingrut W, Avila-Casado C, Chan CT, Crump M, Hogg D, Reich HN. Nephrotic syndrome with cancer immunotherapies: a report of 2 cases. Am J Kidney Dis. 2017;70(4):581–5.
Kobayashi H, Watanabe R, Choyke PL. Improving conventional enhanced permeability and retention (EPR) effects; what is the appropriate target? Theranostics. 2014;4(1):81.
Kobayashi Yoshie, Lim Seung-Oe, Yamaguchi Hirohito. Oncogenic signaling pathways associated with immune evasion and resistance to immune checkpoint inhibitors in cancer. Semin Cancer Biol. 2020;65:51–64.
Kong KF, Fu G, Zhang Y, Yokosuka T, Casas J, Canonigo-Balancio AJ, Becart S, Kim G, Yates JR III, Kronenberg M, Saito T. Protein kinase C-η controls CTLA-4–mediated regulatory T cell function. Nat Immunol. 2014;15(5):465–72.
Kwon ED, Hurwitz AA, Foster BA, Madias C, Feldhaus AL, Greenberg NM, Burg MB, Allison JP. Manipulation of T cell costimulatory and inhibitory signals for immunotherapy of prostate cancer. Proc Natl Acad Sci. 1997;94(15):8099–103.
Lai WY, Huang BT, Wang JW, Lin PY, Yang PC. A novel PD-L1-targeting antagonistic DNA aptamer with antitumor effects. Mol Ther Nucleic Acids. 2016;5: e397.
Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Cowey CL, Lao CD, Schadendorf D, Dummer R, Smylie M, Rutkowski P, Ferrucci PF. Combined nivolumab and Ipilimumab or monotherapy in untreated melanoma. N Engl J Med. 2015;373(1):23–34.
Leach DR, Krummel MF, Allison JP. Enhancement of antitumor immunity by CTLA-4 blockade. Science. 1996;271(5256):1734–6.
Ledford H. Melanoma drug wins US approval. Nature. 2011;471(7340):561.
Lee CH, Lee SH, Kim JH, Noh YH, Noh GJ, Lee SW. Pharmacokinetics of a cholesterol-conjugated aptamer against the hepatitis C virus (HCV) NS5B protein. Mol Ther Nucleic Acids. 2015;4:e254.
Lin Y, Qiu Q, Gill SC, Jayasena SD. Modified RNA sequence pools for in vitro selection. Nucleic Acids Res. 1994;22(24):5229–34.
Maeda TK, Sugiura D, Okazaki IM, Maruhashi T, Okazaki T. Atypical motifs in the cytoplasmic region of the inhibitory immune co-receptor LAG-3 inhibit T cell activation. J Biol Chem. 2019;294(15):6017–26.
Maker AV, Phan GQ, Attia P, Yang JC, Sherry RM, Topalian SL, Kammula US, Royal RE, Haworth LR, Levy C, Kleiner D. Tumor regression and autoimmunity in patients treated with cytotoxic T lymphocyte–associated antigen 4 blockade and interleukin 2: a phase I/II study. Ann Surg Oncol. 2005;12:1005–16.
Marin-Acevedo JA, Dholaria B, Soyano AE, Knutson KL, Chumsri S, Lou Y. Next generation of immune checkpoint therapy in cancer: new developments and challenges. J Hematol Oncol. 2018;11:1–20.
Mellor AL, Chandler P, Baban B, Hansen AM, Marshall B, Pihkala J, Waldmann H, Cobbold S, Adams E, Munn DH. Specific subsets of murine dendritic cells acquire potent T cell regulatory functions following CTLA4-mediated induction of indoleamine 2,3 dioxygenase. Int Immunol. 2004;16(10):1391–401.
Ménard C, Ghiringhelli F, Roux S, Chaput N, Mateus C, Grohmann U, Caillat-Zucman S, Zitvogel L, Robert C. Ctla-4 blockade confers lymphocyte resistance to regulatory T-cells in advanced melanoma: surrogate marker of efficacy of tremelimumab? Clin Cancer Res. 2008;14(16):5242–9.
Mishra AK, Kadoishi T, Wang X, Driver E, Chen Z, Wang XJ, Wang JH. Squamous cell carcinomas escape immune surveillance via inducing chronic activation and exhaustion of CD8+ T Cells co-expressing PD-1 and LAG-3 inhibitory receptors. Oncotarget. 2016;7(49):81341.
Morita Y, Leslie M, Kameyama H, Volk DE, Tanaka T. Aptamer therapeutics in cancer: current and future. Cancers. 2018;10(3):80.
Nakanishi J, Wada Y, Matsumoto K, Azuma M, Kikuchi K, Ueda S. Overexpression of B7–H1 (PD-L1) significantly associates with tumor grade and postoperative prognosis in human urothelial cancers. Cancer Immunol Immunother. 2007;56:1173–82.
Nduom EK, Wei J, Yaghi NK, Huang N, Kong LY, Gabrusiewicz K, Ling X, Zhou S, Ivan C, Chen JQ, Burks JK. PD-L1 expression and prognostic impact in glioblastoma. Neuro Oncol. 2015;18(2):195–205.
Ostrov DA, Shi W, Schwartz JC, Almo SC, Nathenson SG. Structure of murine CTLA-4 and its role in modulating T cell responsiveness. Science. 2000;290(5492):816–9.
Pennock GK, Chow LQ. The evolving role of immune checkpoint inhibitors in cancer treatment. Oncologist. 2015;20(7):812–22.
Phan GQ, Yang JC, Sherry RM, Hwu P, Topalian SL, Schwartzentruber DJ, Restifo NP, Haworth LR, Seipp CA, Freezer LJ, Morton KE. Cancer regression and autoimmunity induced by cytotoxic T lymphocyte-associated antigen 4 blockade in patients with metastatic melanoma. Proc Natl Acad Sci. 2003;100(14):8372–7.
Poggio M, Hu T, Pai CC, Chu B, Belair CD, Chang A, Montabana E, Lang UE, Fu Q, Fong L, Blelloch R. Suppression of exosomal PD-L1 induces systemic antitumor immunity and memory. Cell. 2019;177(2):414–27.
Prodeus A, Abdul-Wahid A, Fischer NW, Huang EH, Cydzik M, Gariépy J. Targeting the PD-1/PD-L1 immune evasion axis with DNA aptamers as a novel therapeutic strategy for the treatment of disseminated cancers. Mol Ther Nucleic Acids. 2015;4: e237.
Pu Y, Ji Q. Tumor-associated macrophages regulate PD-1/PD-L1 immunosuppression. Front Immunol. 2022;13:2004.
Qin S, Xu L, Yi M, Yu S, Wu K, Luo S. Novel immune checkpoint targets: moving beyond PD-1 and CTLA-4. Mol Cancer. 2019;18:1–4.
Ramagopal UA, Liu W, Garrett-Thomson SC, Bonanno JB, Yan Q, Srinivasan M, Wong SC, Bell A, Mankikar S, Rangan VS, Deshpande S. Structural basis for cancer immunotherapy by the first-in-class checkpoint inhibitor ipilimumab. Proc Natl Acad Sci. 2017;114(21):E4223–32.
Ribas A, Comin-Anduix B, Economou JS, Donahue TR, de la Rocha P, Morris LF, Jalil J, Dissette VB, Shintaku IP, Glaspy JA, Gomez-Navarro J. Intratumoral immune cell infiltrates, FoxP3, and indoleamine 2, 3-dioxygenase in patients with melanoma undergoing CTLA4 blockade. Clin Cancer Res. 2009;15(1):390–9.
Riccardi C, Musumeci D, Krauss IR, Piccolo M, Irace C, Paduano L, Montesarchio D. Exploring the conformational behaviour and aggregation properties of lipid-conjugated AS1411 aptamers. Int J Biol Macromol. 2018;118:1384–99.
Rohatgi Anjali, Massa Ryan Campbell, Gooding William E, Bruno Tullia C, Vignali Dario, Kirkwood John M. A phase II study of anti-PD1 monoclonal antibody (Nivolumab) administered in combination with anti-LAG3 monoclonal antibody (Relatlimab) in patients with metastatic melanoma naive to prior immunotherapy in the metastatic setting. J Clin Oncol. 2020;38:TPS10085–TPS10085.
Ruckman J, Green LS, Beeson J, Waugh S, Gillette WL, Henninger DD, Claesson-Welsh L, Janjic N. 2′-Fluoropyrimidine RNA-based aptamers to the 165-amino acid form of vascular endothelial growth factor (VEGF165): inhibition of receptor binding and VEGF-induced vascular permeability through interactions requiring the exon 7-encoded domain. J Biol Chem. 1998;273(32):20556–67.
Ruffo E, Wu RC, Bruno TC, Workman CJ, Vignali DA. Lymphocyte-activation gene 3 (LAG3): the next immune checkpoint receptor. Semin Immunol. 2019;42:101305.
Sabatos CA, Chakravarti S, Cha E, Schubart A, Sánchez-Fueyo A, Zheng XX, Coyle AJ, Strom TB, Freeman GJ, Kuchroo VK. Interaction of Tim-3 and Tim-3 ligand regulates T helper type 1 responses and induction of peripheral tolerance. Nat Immunol. 2003;4(11):1102–10.
Sanderson K, Scotland R, Lee P, Liu D, Groshen S, Snively J, Sian S, Nichol G, Davis T, Keler T, Yellin M. Autoimmunity in a phase I trial of a fully human anti-cytotoxic T-lymphocyte antigen-4 monoclonal antibody with multiple melanoma peptides and Montanide ISA 51 for patients with resected stages III and IV melanoma. J Clin Oncol. 2005;23(4):741–50.
Santulli-Marotto S, Nair SK, Rusconi C, Sullenger B, Gilboa E. Multivalent RNA aptamers that inhibit CTLA-4 and enhance tumor immunity. Can Res. 2003;63(21):7483–9.
Schneider H, Martin M, Agarraberes FA, Yin L, Rapoport I, Kirchhausen T, Rudd CE. Cytolytic T lymphocyte-associated antigen-4 and the TCRζ/CD3 complex, but not CD28, interact with clathrin adaptor complexes AP-1 and AP-2. J Immunol. 1999;163(4):1868–79.
Smith-Garvin JE, Koretzky GA, Jordan MS. T cell activation. Annu Rev Immunol. 2009;27:591–619.
Soldevilla MM, Hervas S, Villanueva H, Lozano T, Rabal O, Oyarzabal J, Lasarte JJ, Bendandi M, Inoges S, López-Díaz de Cerio A, Pastor F. Identification of LAG3 high affinity aptamers by HT-SELEX and conserved motif accumulation (CMA). PLoS ONE. 2017;12(9):e0185169.
Song P, Zhang D, Cui X, Zhang L. Meta-analysis of immune-related adverse events of immune checkpoint inhibitor therapy in cancer patients. Thorac Cancer. 2020;11(9):2406–30.
Sosman JA, Moon J, Tuthill RJ, Warneke JA, Vetto JT, Redman BG, Liu PY, Unger JM, Flaherty LE, Sondak VK. A phase 2 trial of complete resection for stage IV melanoma: results of Southwest Oncology Group Clinical Trial S9430. Cancer. 2011;117(20):4740–6.
Ștefan G, Hosu O, De Wael K, Lobo-Castañón MJ, Cristea C. Aptamers in biomedicine: selection strategies and recent advances. Electrochim Acta. 2021;376: 137994.
Tang SQ, Tang LL, Mao YP, Li WF, Chen L, Zhang Y, Guo Y, Liu Q, Sun Y, Xu C, Ma J. The pattern of time to onset and resolution of immune-related adverse events caused by immune checkpoint inhibitors in cancer: a pooled analysis of 23 clinical trials and 8,436 patients. Cancer Res Treat. 2021;53(2):339–54.
Thompson CB, Allison JP. The emerging role of CTLA-4 as an immune attenuator. Immunity. 1997;7(4):445–50.
Thompson RH, Gillett MD, Cheville JC, Lohse CM, Dong H, Webster WS, Krejci KG, Lobo JR, Sengupta S, Chen L, Zincke H. Costimulatory B7–H1 in renal cell carcinoma patients: indicator of tumor aggressiveness and potential therapeutic target. Proc Natl Acad Sci. 2004;101(49):17174–9.
Triebel F, Jitsukawa S, Baixeras E, Roman-Roman S, Genevee C, Viegas-Pequignot E, Hercend T. LAG-3, a novel lymphocyte activation gene closely related to CD4. J Exp Med. 1990;171(5):1393–405.
Tuerk C, Gold L. Systematic evolution of ligands by exponential enrichment: RNA ligands to bacteriophage T4 DNA polymerase. Science. 1990;249(4968):505–10.
Vonderheide RH, LoRusso PM, Khalil M, Gartner EM, Khaira D, Soulieres D, Dorazio P, Trosko JA, Rüter J, Mariani GL, Usari T. Tremelimumab in combination with exemestane in patients with advanced breast cancer and treatment-associated modulation of inducible costimulator expression on patient T cells. Clin Cancer Res. 2010;16(13):3485–94.
Willis MC, Collins B, Zhang T, Green LS, Sebesta DP, Bell C, Kellogg E, Gill SC, Magallanez A, Knauer S, Bendele RA. Liposome-anchored vascular endothelial growth factor aptamers. Bioconjug Chem. 1998;9(5):573–82.
Wolchok JD, Neyns B, Linette G, Negrier S, Lutzky J, Thomas L, Waterfield W, Schadendorf D, Smylie M, Guthrie T, Grob JJ. Ipilimumab monotherapy in patients with pretreated advanced melanoma: a randomised, double-blind, multicentre, phase 2, dose-ranging study. Lancet Oncol. 2010;11(2):155–64.
Wu X, Li F, Li Y, Yu Y, Liang C, Zhang B, Zhao C, Lu A, Zhang G. A PD-L1 aptamer selected by loss-gain cell-SELEX conjugated with paclitaxel for treating triple-negative breast cancer. Med Sci Monit. 2020;26:e925583–91.
Xu B, Yuan L, Gao Q, Yuan P, Zhao P, Yuan H, Fan H, Li T, Qin P, Han L, Fang W. Circulating and tumor-infiltrating Tim-3 in patients with colorectal cancer. Oncotarget. 2015;6(24):20592.
Yamazaki T, Akiba H, Iwai H, Matsuda H, Aoki M, Tanno Y, Shin T, Tsuchiya H, Pardoll DM, Okumura K, Azuma M. Expression of programmed death 1 ligands by murine T cells and APC. J Immunol. 2002;169(10):5538–45.
Yang Ling, Ning Qian, Tang Sheng-song. Recent advances and next breakthrough in immunotherapy for cancer treatment. J Immunol Res. 2022;2022:1–9.
Yao F, An Y, Lai X, Li X, Yu Z, Yang XD. Novel nanotherapeutics for cancer immunotherapy by CTLA-4 aptamer-sfunctionalised albumin nanoparticle loaded with antihistamine. J Cancer Res Clin Oncol. 2023. https://doi.org/10.1007/s00432-023-04698-y.
Yu X, Huang X, Chen X, Liu J, Wu C, Pu Q, Wang Y, Kang X, Zhou L. Characterization of a novel anti-human lymphocyte activation gene 3 (LAG-3) antibody for cancer immunotherapy. MAbs. 2019;11(6):1139–48.
Zhao F, Zhu J, Yu R, Shao T, Chen S, Zhang G, Shu Q. Cutaneous adverse events in patients treated with PD-1/PD-L1 checkpoint inhibitors and their association with survival: a systematic review and meta-analysis. Sci Rep. 2022;12(1):20038.
Zhao Y, Harrison DL, Song Y, Ji J, Huang J, Hui E. Antigen-presenting cell-intrinsic PD-1 sneutralises PD-L1 in cis to attenuate PD-1 signaling in T cells. Cell Rep. 2018;24(2):379–90.
Zhao Y, Lee CK, Lin CH, Gassen RB, Xu X, Huang Z, Xiao C, Bonorino C, Lu LF, Bui JD, Hui E. PD-L1: CD80 cis-heterodimer triggers the costimulatory receptor CD28 while repressing the inhibitory PD-1 and CTLA-4 pathways. Immunity. 2019;51(6):1059–73.
Zhou G, Noordam L, Sprengers D, Doukas M, Boor PP, van Beek AA, Erkens R, Mancham S, Grünhagen D, Menon AG, Lange JF. Blockade of LAG3 enhances responses of tumour-infiltrating T cells in mismatch repair-proficient liver metastases of colorectal cancer. Oncoimmunology. 2018;7(7): e1448332.
Zhou J, Rossi J. Aptamers as targeted therapeutics: current potential and challenges. Nat Rev Drug Discov. 2017;16(3):181–202.
Zhou J, Soontornworajit B, Martin J, Sullenger BA, Gilboa E, Wang Y. A hybrid DNA aptamer–dendrimer nanomaterial for targeted cell labeling. Macromol Biosci. 2009;9(9):831–5.
Zhou F, Fu T, Huang Q, Kuai H, Mo L, Liu H, Wang Q, Peng Y, Han D, Zhao Z, Fang X. Hypoxia-activated PEGylated conditional aptamer/antibody for cancer imaging with improved specificity. J Am Chem Soc. 2019;141(46):18421–7.
Zhu G, Chen X. Aptamer-based targeted therapy. Adv Drug Deliv Rev. 2018;134:65–78.
Funding
This research received no specific grant from public, commercial, or not-for-profit funding agencies.
Author information
Authors and Affiliations
Contributions
Both authors have contributed to this manuscript, and KTR gave the outline of it. Later, the data collection and manuscript preparation was done by PKM. KTR did the final part and evaluation. Both authors commented on and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interests
The authors report no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kejamurthy, P., Devi, K.T.R. Immune checkpoint inhibitors and cancer immunotherapy by aptamers: an overview. Med Oncol 41, 40 (2024). https://doi.org/10.1007/s12032-023-02267-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12032-023-02267-4