Abstract
The CYLD gene is a tumor suppressor, reduced in many cancers. Here, we aimed to investigate CYLD protein level and NF-κβ/TNF-α signaling pathway in rectal cancer patients with Lactobacillus acidophilus (L. acidophilus) consumption. One hundred ten patients with non-metastatic rectal cancer were randomly divided into L. acidophilus probiotic (500 mg, three times daily) and placebo groups for 13 weeks. The expression of CYLD, TNF-α, and NF-κB proteins and the genes involved in the NF-κβ/TNF-α pathway were evaluated using ELISA and qPCR techniques. The survival rate was measured after five years. Unlike the placebo group, the results showed a significant increase in the expression of CYLD protein and tumor suppressor genes, including FOXP3, ROR-γ, Caspase3, GATA3, T-bet, and a considerable decrease in the expression of NF-ҝβ and TNF-α proteins and oncogenes, including STAT3, 4, 5, 6, and SMAD 3, in the probiotic group. A higher overall survival rate was seen after L. acidophilus consumption compared to the placebo group (P < 0.05). L. acidophilus consumption can reduce inflammation factors by affecting CYLD protein and its downstream signaling pathways.
Graphical abstract
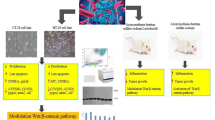
A schematic plot of probiotic consumption Effects on the CYLD protein in regulating the NF-ĸβ signaling pathway in colorectal cancer. NF-ĸβ can be activated by canonical and noncanonical pathways, which rely on IκB degradation and p100 processing, respectively. In the canonical NF-κβ pathway, dimmers, such as p65/p50, are maintained in the cytoplasm by interacting with an IκBα protein. The binding of a ligand to a cell-surface receptor activates TRAF2, which triggers an IKK complex, containing -α, -β, -g, which phosphorylates IKK-β. It then phosphorylates IκB-α, leading to K48-ubiquitination and degradation of this protein. The p65/p50 protein freely enters the nucleus to turn on target genes. The non-canonical pathway is primarily involved in p100/RelB activation. It differs from the classical pathway in that only certain receptor signals activate this pathway. It proceeds through an IKK complex that contains two IKK-α subunits but not NEMO. Several materials including peptidoglycan, phorbol, myristate, acetate, and gram-positive bacteria such as probiotics inhibit NF-κB by inducing CYLD. This protein can block the canonical and noncanonical NF-κβ pathways by removing Lys-63 ubiquitinated chains from activated TRAFs, RIP, NEMO, and IKK (α, β, and γ). Moreover, TNF-α induces apoptosis by binding caspase-3 to FADD.



Similar content being viewed by others
Data availability
The data supporting this study’s findings are available from the corresponding author, AMA, upon reasonable request.
References
Sivamaruthi BS, Kesika P, Chaiyasut C. The role of probiotics in colorectal cancer management. Evid-Based Complement Altern Med. 2020. https://doi.org/10.1155/2020/3535982.
Molska M, Reguła J. Potential mechanisms of probiotics action in the prevention and treatment of colorectal cancer. Nutrients. 2019;11(10):2453.
Daniluk U. Probiotics, the new approach for cancer prevention and/or potentialization of anti-cancer treatment. J Clin Exp Oncol. 2012;1:2.
Wollowski I, Rechkemmer G, Pool-Zobel BL. Protective role of probiotics and prebiotics in colon cancer. Am J Clin Nutr. 2001;73(2):451s-s455.
Lei H, et al. Deubiquitinases in hematological malignancies. Biomarker Research. 2021;9(1):1–23.
Terzić J, et al. Inflammation and colon cancer. Gastroenterology. 2010;138(6):2101-2114.e5.
Jono H, et al. NF-κB is essential for induction of CYLD, the negative regulator of NF-κB: evidence for a novel inducible autoregulatory feedback pathway. J Biol Chem. 2004;279(35):36171–4.
Pikarsky E, et al. NF-κB functions as a tumour promoter in inflammation-associated cancer. Nature. 2004;431(7007):461–6.
Liang H-L, et al. MiR-454 prompts cell proliferation of human colorectal cancer cells by repressing CYLD expression. Asian Pac J Cancer Prev. 2015;16(6):2397–402.
Sun S. CYLD: a tumor suppressor deubiquitinase regulating NF-κB activation and diverse biological processes. Cell Death Differ. 2010;17(1):25–34.
Khodaii Z, et al. Novel targets in rectal cancer by considering lncRNA–miRNA–mRNA network in response to Lactobacillus acidophilus consumption: a randomized clinical trial. Sci Rep. 2022;12(1):1–8.
Mego M, et al. Probiotic bacteria in cancer patients undergoing chemotherapy and radiation therapy. Complement Ther Med. 2013;21(6):712–23.
Rondanelli M, et al. Using probiotics in clinical practice: where are we now? A review of existing meta-analyses. Gut microbes. 2017;8(6):521–43.
Demers M, Dagnault A, Desjardins J. A randomized double-blind controlled trial: impact of probiotics on diarrhea in patients treated with pelvic radiation. Clin Nutr. 2014;33(5):761–7.
Flesch AT, et al. Perioperative synbiotics administration decreases postoperative infections in patients with colorectal cancer: a randomized, double-blind clinical trial. Rev Col Bras Cir. 2017;44:567–73.
Burns MB, et al. Virulence genes are a signature of the microbiome in the colorectal tumor microenvironment. Genome medicine. 2015;7(1):1–2.
Zitvogel L, et al. The microbiome in cancer immunotherapy: diagnostic tools and therapeutic strategies. Science. 2018;359(6382):1366–70.
Gianotti L, et al. A randomized double-blind trial on perioperative administration of probiotics in colorectal cancer patients. World J Gastroenterol: WJG. 2010;16(2):167.
Karin M, et al. NF-κB in cancer: from innocent bystander to major culprit. Nat Rev Cancer. 2002;2(4):301–10.
Costello CM, et al. Dissection of the inflammatory bowel disease transcriptome using genome-wide cDNA microarrays. PLoS Med. 2005;2(8): e199.
Rutter M, et al. Severity of inflammation is a risk factor for colorectal neoplasia in ulcerative colitis. Gastroenterology. 2004;126(2):451–9.
Trompouki E, et al. CYLD is a deubiquitinating enzyme that negatively regulates NF-κB activation by TNFR family members. Nature. 2003;424(6950):793–6.
Bullock M, et al. FOXO3 expression during colorectal cancer progression: biomarker potential reflects a tumour suppressor role. Br J Cancer. 2013;109(2):387–94.
Wang W, et al. Overexpressed GATA3 enhances the sensitivity of colorectal cancer cells to oxaliplatin through regulating MiR-29b. Cancer Cell Int. 2020;20(1):1–6.
Dariya B, et al. Targeting STAT proteins via computational analysis in colorectal cancer. Mol Cell Biochem. 2021;476(1):165–74.
Spano J-P, et al. JAK/STAT signalling pathway in colorectal cancer: a new biological target with therapeutic implications. Eur J Cancer. 2006;42(16):2668–70.
Koveitypour Z, et al. Signaling pathways involved in colorectal cancer progression. Cell Biosci. 2019;9(1):1–4.
Fang T, et al. Prognostic role and clinicopathological features of SMAD4 gene mutation in colorectal cancer: a systematic review and meta-analysis. BMC Gastroenterol. 2021;21(1):1–2.
Funding
This research was funded by the Iran University of Medical Sciences (IUMS) (Grant No. 36001) and the Tehran University of Medical Sciences (Grant No. 29811).
Author information
Authors and Affiliations
Contributions
FZ: contributed to study conception and manuscript preparation. SK: contributed to sample processing, data analysis, and manuscript preparation. MRK: contributed to manuscript preparation. AP and TA: contributed to manuscript revision. PH: contributed to sample collection and processing. EE, MA, and AN: contributed to sample collection. AMA: contributed to study conception and design and manuscript revision.
Corresponding author
Ethics declarations
Conflict of interest
The manuscript authors have no conflicts of interest to declare and are responsible for the paper’s content.
Ethical approval
All methods were performed under the relevant guidelines and regulations. All procedures performed in studies involving human participants were in accordance with the institutional and/or national research committee ethical standards. The experimental procedures and care protocols were approved by a review board committee of the Tehran University of Medical Sciences (IR.TUMS.VCR.REC.1397.733) and the Iran University of Medical Sciences (IR.IUMS.REC.1398.548) and registered by the Iranian Randomized Control Trial (IRCT) ethical board (NO: IRCT2014092118745N3). Written informed consent was obtained from each participant before sample collection.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zamani, F., Khalighfard, S., Kalhori, M.R. et al. Expanding CYLD protein in NF-κβ/TNF-α signaling pathway in response to Lactobacillus acidophilus in non-metastatic rectal cancer patients. Med Oncol 40, 302 (2023). https://doi.org/10.1007/s12032-023-02170-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12032-023-02170-y