Abstract
Engraftment syndrome (ES) is a non-infectious complication seen both in autologous and allogeneic hematopoietic stem cell transplants and is characterized by the presence of non-infectious fever, diarrhea, skin rash, pulmonary infiltration, pulmonary edema, and deranged renal and liver function tests This review will be delineating the incidence of ES, important differential diagnoses to be considered and management options. The literature search was done through various databases like PubMed, Google scholar, Cochrane library, and EMBASE. The incidence of engraftment syndrome was ranging from 8 to 50% in patients undergoing Autologous stem cell transplantation while the incidence was 10–77% in patients undergoing Allogeneic stem cell transplantation. Fever was the most commonly observed symptom of ES in both Autologous and Allogeneic stem cell transplantation while the second most frequently reported symptom was non-infectious diarrhea in patients undergoing autologous stem cell transplantation and Skin rash in patients with Allogeneic stem cell transplantation. Pro-inflammatory cytokines and immune response dysregulation were highlighted as the mechanism behind ES development. The significant difference between ES and aGVHD was observed based on cytokines, with IL-12, IL-1β, IL-6, TNF-α, and IFN-γ levels in plasma being higher in patients with ES as compared to patients with aGVHD. Intravenous methylprednisolone was used as the treatment of choice in the majority of the studies. Overall the incidence of ES was high in patients undergoing allogeneic hematopoietic stem cells transplantation. The survival in patients developing ES was less compared to those who did not develop ES. Engraftment syndrome is one of the complications following hematopoietic stem cell transplantation that need early identification, differentiation from infectious complications, and aGVHD and timely initiation of corticosteroids therapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hematopoietic stem cell transplantation (HSCT) is a medical procedure that is commonly used as an excellent resort for the treatment of various malignancies by infusing stem cells following chemotherapy or radiotherapy [1]. E. Donnall Thomas carried out the first procedure of HSCT in 1957; this was regarded as a revolutionary step toward therapeutic advances in cancer management [2]. According to recent statistics in the year 2019 by World Health Organization (WHO), about 50,000 HSCT procedures are being performed annually [3]. Two types of HSCT have been described, one is autologous stem cell transplantation (Auto-SCT) which uses the recipient stem cells, and the other is allogeneic stem cell transplantation (Allo-SCT) which uses the stem cells from matched or unrelated human leukocyte antigen (HLA) compatible donors [4]. Though HSCT is not only paving the way toward advanced management of various malignant and benign diseases but also posing some significant adverse events and engraftment syndrome is one of the serious side effect profiles associated with HSCT.
Engraftment syndrome is a group of clinical signs and symptoms associated with the process of neutrophil recovery after HSCT [5]. Engraftment syndrome presented with symptoms like non-infectious fever, rash, pulmonary infiltration, or edema that are found to be in close association with HSCT outcome measures [6]. Though some patients developed limited featured ES it has also been associated with transplant-related mortalities [7]. A strong association between ES and acute graft vs host reaction (aGVHD) has been studied, but the cytokine profile is suitable enough to allow the differentiation between these two entities. So, hypothesizing that aGVHD and ES are two different disorders would be of great value to the literature and future perspectives related to ES [8].
We aim this review to summarize the ES incidence, pathogenesis, diagnostic and therapeutic profile of ES, along with aGVHD differentiation from ES in HSCT. This review will also aim to highlight the signs and symptoms variation of ES between Autologous and Allogeneic stem cell transplantation with various therapeutic approaches and benefits in ES.
Materials and methods
Search strategies
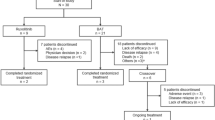
This study was conducted following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [9]. A comprehensive literature search was done from July 21, 2022, to September 22, 2022. The literature search for this systematic review was done through various databases like PubMed, Google scholar, Web of Science, EMBASE, and Cochrane database by using MeSH key terms of engraftment syndrome (ES), Autologous stem cell transplantation (ASCT), Allogeneic stem cell transplantation, and Hematopoietic stem cell transplantation (HSCT), Graft vs host disease (GVHD). After careful consideration of inclusion and exclusion criteria, a total of 12 studies were included to synthesize this systematic review, and studies involving the occurrence of ES in both Autologous stem cell transplantation or Allogeneic stem cell transplantation were included.
Inclusion and exclusion criteria
This study will include studies involving patients of both adult and pediatric age groups having different types of hematological and non-hematological malignancies undergoing Autologous stem cell transplantation (Auto-SCT) and Allogeneic stem cell transplantation (Allo-SCT) for any of the disease either hematological malignancies or non-hematological benign and malignant disorders. The studies related to ES from past twenty years were included. The studies in which patients were undergoing conventional chemotherapeutic treatment were excluded and those lacking follow-up after ASCT or allogeneic stem cell transplantation were also excluded.
Quality assessment of studies
The quality assessment of involved studies was done by two independent reviewers selected based on competency in the field of research. For quality assessment Newcastle–Ottawa scale (NOS) and Jadad five-item scale was used and studies like RCT, meta-analysis, systematic reviews, case–control, and cohort studies were included while short Communications, letter to the editor, commentaries, unpublished articles, and studies with language other than English were excluded. Studies with a score ≤ 4 (low quality) were excluded while studies with a score ≥ 6 (high quality) were included for the synthesis of this systematic review.
Data extraction
Data extraction was done independently by two investigators and studies showing the association or occurrence of ES after autologous and Allogeneic stem cell transplantation were selected. The data extraction regarding study name, year of study, type of study, country of origin, ES incidence, ES diagnosis, ES treatment, signs and symptoms of ES, and finally conditioning regimens and aGVHD prophylactic treatment used in Allogeneic stem cell transplantation were extracted and data was arranged in tabulated configuration (Fig. 1).
Diagnostic criteria leading to engraftment syndrome diagnosis
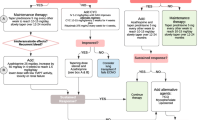
To date, various diagnostic criteria have been proposed to simplify the correct definition of ES, however; Spitzer [5] and Maiolino [10] criteria are the most commonly used criteria to define ES in clinical settings. The presence of non-infectious fever (38 °C), non-infectious diarrhea having 2 or more episodes, and Maculo-papular exanthema rash involving over 25% of body surface area, were the common fractures of ES between Spitzer and Maiolino criteria. However, pulmonary edema of non-cardiogenic origin, weight gain of over 2.5% of the basal level, deranged liver function tests (bilirubin ≥ 2 mg/100 ml and ALT and AST 2 time of the normal), deranged Renal function tests (creatinine two times of the normal value), and Transient encephalopathy of unknown origin was solely described in Spitzer criteria as compared to Maiolino criteria. The flow sheet of Engraftment syndrome criteria is given in Fig. 2 below.
Results
Engraftment syndrome (ES) is a well-known complication that is found to be associated with Hematopoietic stem cell transplantation (Auto-HSCT and Allo-HSCT) that is manifested through a set of clinically significant signs and symptoms like non-infectious fever, non-infectious diarrhea, skin rash, pulmonary infiltration or edema, weight gain and deranged RFTs, and LFTs [11]. The exact pathophysiological mechanism leading to ES is still unclear however; studies have evaluated the association of various pro-inflammatory cytokines such as IL-2, IL-6, IL-8, INF, and TNF-alpha with the development of ES following hematopoietic stem cell transplantation [12]. ES is also characterized as a constellating set of symptoms occurring during the recovery of neutrophils following Autologous and Allogeneic stem cell transplantation. The commutative incidence of ES following hematopoietic stem cell transplantation has been reported as 5% to 75% as reported in various studies [14,15,15] (Figs. 3 and 4).
Engraftment syndrome following autologous and allogeneic stem cell transplantation
Discussion
Engraftment syndrome (ES) is a well-known complication that is found to be associated with Hematopoietic stem cell transplantation (Auto-HSCT and Allo-HSCT) that is manifested through a set of clinically significant signs and symptoms. Engraftment syndrome (ES) is a non-infectious complication seen both in autologous and allogeneic hematopoietic stem cell transplants and is characterized by the presence of non-infectious fever, diarrhea, skin rash, pulmonary infiltration, pulmonary edema, and deranged renal and liver function tests.
Pathophysiological mechanism of engraftment syndrome
Various animal model studies and human model studies have been performed to elaborate on the exact mechanism of ES, still very unclear to label the exact mechanism of ES. However, various human model studies have shown the role of the immune system in the development of ES even in Autologous stem cell transplantation or even in HLA-absent or minor histocompatibility mismatch cases [16]. Various studies have delineated the role of various pro-inflammatory cytokines like IL-1, TNFα, IFN-γ, and IL-12 along with immune system dysregulation [17]. However, studies have also evaluated the role of various other cytokines profiles that were found to be high in isolated ES as compared to aGVHD cytokine profiles including IL-1β, IL-6, IL-12, IL-4, and IL-13 [18]. The presence of higher levels of IL-1β in ES was a leading point toward the association of inflammasome-mediated inflammation and ES development. A proposed hypothetical model involving the exact pathophysiological Mechanism of ES was cytokine-mediated enhanced antigen presentation to T-cells with enhanced T-cells activation and graft rejection in allogeneic settings and reduced tolerance in the autologous setting. With reduced effects of regulatory T-cell (Treg) functions, the T-cells destined to recognize self-MHC and self-peptides become Cytotoxic T-cells with tissue destruction and ultimately graft rejection and ES [16]. There is still more work pending to elaborate the exact mechanism of ES while considering the hypothetical role of various cytokines and immune system dysregulation with reduced Treg functions.
Engraftment syndrome (ES) vs acute Graft-vs-Host disease (aGVHD) and differentiating role of cytokines
There have been a lot of discussions to differentiate between ES and aGVHD, and various studies have tried to explain this difference and got fruitful results, but how these two terms are different and which pathophysiological Mechanism is involved in both ES and aGVHD it’s still very unclear. However, with the progress in this field, studies have shown the difference in inflammatory and immunological responses between ES and aGVHD. A study by Khandelwal et al., involving the pediatric population has shown the difference of various pro-inflammatory cytokines in the development of isolated ES and isolated aGVHD, which was showing the significant difference of inflammatory response in terms of pro-inflammatory cytokines between aGVHD and isolated ES with higher plasma concentrations of IL-12, IL-1β, IL-6, TNFα, and IFN-γ in patients with ES as compared to aGVHD when measured at day zero to week 8 following Hematopoietic stem cell transplantation [19]. Similarly, a study by Konuma et al. was also showing higher plasma levels of IL-6, IL-12, TNFα, and IFN-γ in patients with engraftment syndrome validating the role of pro-inflammatory cytokines in the development of ES as compared to aGVHD which also shows higher levels of cytokines but not higher than ES [20].
Management profile of engraftment syndrome (ES) and acute graft-vs-host disease (aGVHD)
The management strategies of ES are mainly based on corticosteroid-based treatment, which is started based on a diagnosis of ES while ruling out the other potential causes of clinical symptomatology. A corticosteroid-based therapy is used widely in the effective treatment of ES either following Autologous hematopoietic stem cell transplantation or Allogeneic Hematopoietic stem cell transplantation. The initiation of methylprednisolone 1–1.5 mg/kg/day until the symptoms are resolving; which typically occurs within 2–3 days, followed by a reduction to 40–50 mg PO Prednisone/day for 2–3 days which typically occurs within 2–3 days is considered a good treatment strategy to mitigate the devastating effects of ES [21]. Similarly, studies have evaluated that early initiation of corticosteroid therapy is associated significantly with a reduction in disease progression and severity. According to Sheth et al., early initiation of methylprednisolone 1 mg/kg/day for 3 days while tapering the dose to 0.5 mg/kg/day over 5–7 days was significantly associated with a reduction in ES-related complications and early recovery [22].
In the same vein, the association of ES with aGVHD in patients undergoing Allogeneic Hematopoietic stem cell transplantation is also a factor leading to post-transplantation complications. The utilization of aGVHD prophylaxis is of paramount significance to prevent transplant-related rejections and mortalities. While knowing the role of immune response dysregulation involving effector T-cells, various prophylactic treatment options have revolutionized the prevention of ES/aGVHD [23]. Using T-cell suppression effect through Tacrolimus (Tac) and Cyclosporine (Cys) in combination with methotrexate (MTX) and mycophenolate mofetil (MMF) are the best-known regimens used to prevent aGVHD. According to two RCTs conducted in 1990, the combination of Tac/MTX was the most effective combination used for the treatment of grade II and grade III GVHD as compared to Cys/MTX combination [24, 25].
There are various other treatment strategies now become available for the effective management of GVHD grade II–IV. New advances in aGVHD include the utilization of post-transplant cyclophosphamide at doses of 50 mg/kg on days + 3 and + 4 following the infusion of haploidentical stem cells was associated with a reduction in aGVHD [26]. Similarly, anti-thymocyte globulin (ATG) [27], sirolimus (a mTOR inhibitor) [28], along with select and pan T-cells depletion strategies are now proving fruitful, however; using Tac/MTX/MFM and Cys/MTX/MFM are still considered standard regimens in the prophylaxis of aGVHD [29]. All these strategies help in preventing the hyperactivity of the innate immune system with a significant reduction in cases of ES and aGVHD with improved overall survival (OS) following hematopoietic stem cell transplantation.
Conclusion
Engraftment Syndrome and acute Graft-vs-Host disease are the commonly encountered complications after hematopoietic stem cell transplantation. The mechanism involving these complications is hyperactivity of the innate immune system and pro-inflammatory cytokines storm that predisposed the patients to develop ES/aGVHD following Hematopoietic stem cell transplantation. In this review synthesis, the most common presentation of ES was non-infectious fever, diarrhea, and skin rash following Autologous hematopoietic stem cell transplantation non-infectious fever, and skin rash followed by weight gain was most commonly observed ES presentation after Allogeneic stem cell transplantation. Variable values of ES incidence were observed in this review ranging from 8 to 77% while using the Spitzer and Maiolino criteria of ES. The therapeutic use of corticosteroids, particularly intravenous methylprednisolone at a higher starting dose and followed by tapering, was highly effective in combating ES. Similarly, the use of immunosuppressive therapy was also highly effective in combating aGVHD. The difference between ES and aGVHD was evaluated based on plasma concentrations of various pro-inflammatory cytokines, which were present in higher concentrations in patients with ES as compared to aGVHD patients.
Data availability
All the data generated or analyzed during this study are included in this manuscript.
Abbreviations
- MM:
-
Multiple Myeloma
- HL:
-
Hodgkin Lymphoma
- NHL:
-
Non-Hodgkin Lymphoma
- CLL:
-
Chronic Lymphoblastic Leukaemia
- POEMS:
-
Polyneuropathy, Organomegaly, Endocrinopathy, Monoclonal protein, Skin changes
- ASCT:
-
Autologous Stem Cell Transplantation
- ES:
-
Engraftment syndrome
- CML:
-
Chronic Myeloid Leukaemia
- AML:
-
Acute myeloid leukemia
- ALL:
-
Acute lymphoblastic leukemia
- MDS:
-
Myelodysplastic syndrome
- MF:
-
Myelofibrosis
- MPD:
-
Myeloproliferative disorder
- MAC:
-
Myeloablative conditioning
- RIC:
-
Reduced-intensity conditioning
- TE:
-
Transient encephalopathy
- PE:
-
Pulmonary edema
- LFTs:
-
Liver Function Tests
- RFTs:
-
Renal Function Tests
- Tac:
-
Tacrolimus
- CsA:
-
Cyclosporine
- MMF:
-
Mycophenolate mofetil
- MTX:
-
Methotrexate
- NMAC:
-
Non-myeloablative conditioning
- MEL:
-
Melphalan
- BU:
-
Busulfan
- ATG:
-
Anti-thymocyte globulin
- TBI:
-
Total body irradiation
- FLU:
-
Fludarabine
- STR:
-
Steroid
References
Bazinet A, Popradi G. A general practitioner’s guide to hematopoietic stem-cell transplantation. Curr Oncol. 2019;26(3):187–91. https://doi.org/10.3747/co.26.5033.
Thomas ED, Lochte HL Jr, Lu WC, Ferrebee JW. Intravenous infusion of bone marrow in patients receiving radiation and chemotherapy. N Engl J Med. 1957;257(11):491–6. https://doi.org/10.1056/NEJM195709122571102.
World Health Organization (WHO) Haematopoietic Stem Cell Transplantation HSCtx [Web page] Geneva, Switzerland: WHO; 2018. https://www.who.int/transplantation/hsctx/en.
Kanakry CG, Fuchs EJ, Luznik L. Modern approaches to HLA-haploidentical blood or marrow transplantation. Nat Rev Clin Oncol. 2016;13(2):132. https://doi.org/10.1038/nrclinonc.2015.234.
Spitzer TR. Engraftment syndrome following hematopoietic stem cell transplantation. Bone Marrow Transplant. 2001;27(9):893–8. https://doi.org/10.1038/sj.bmt.1703015.
Spitzer TR. Engraftment syndrome: double-edged sword of hematopoietic cell transplants. Bone Marrow Transplant. 2015;50(4):469–75. https://doi.org/10.1038/bmt.2014.296.
Ravoet C, Feremans W, Husson B, et al. Clinical evidence for an engraftment syndrome associated with early and steep neutrophil recovery after autologous blood stem cell transplantation. Bone Marrow Transplant. 1996;18(5):943–7.
Schmid I, Stachel D, Pagel P, Albert MH. Incidence, predisposing factors, and outcome of engraftment syndrome in pediatric allogeneic stem cell transplant recipients. Biol Blood Marrow Transplant. 2008;14(4):438–44. https://doi.org/10.1016/j.bbmt.2008.02.002.
Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group*. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151(4):264–9.
Maiolino A, Biasoli I, Lima J, Portugal AC, Pulcheri W, Nucci M. Engraftment syndrome following autologous hematopoietic stem cell transplantation: definition of diagnostic criteria. Bone Marrow Transplant. 2003;31(5):393–7. https://doi.org/10.1038/sj.bmt.1703855.
Sheth V, Jain R, Gore A, Ghanekar A, Saikia T. Engraftment syndrome: clinical features and predictive factors in autologous stem cell transplant. Indian J Hematol Blood Transfus. 2018;34(3):448–53.
Rabinowitz J, Petros WP, Stuart AR, Peters WP. Characterization of endogenous cytokine concentrations after high-dose chemotherapy with autologous bone marrow support. Blood. 1993;81(9):2452–9.
Cahill RA, Spitzer TR, Mazumder A. Marrow engraftment and clinical manifestations of capillary leak syndrome. Bone Marrow Transplant. 1996;18(1):177–84.
Madero L, Vicent MG, Sevilla J, Prudencio M, Rodríguez F, Díaz MA. Engraftment syndrome in children undergoing autologous peripheral blood progenitor cell transplantation. Bone Marrow Transplant. 2002;30(6):355–8. https://doi.org/10.1038/sj.bmt.1703645.
Grant A, Chapman LRM, Mitchell R, O’Brien TA. Engraftment syndrome following hematopoietic stem cell transplant: a review of the literature. Clin Transplant. 2020;34(6):e13875. https://doi.org/10.1111/ctr.13875.
Cornell RF, Hari P, Drobyski WR. Engraftment syndrome after autologous stem cell transplantation: an update unifying the definition and management approach. Biol Blood Marrow Transplant. 2015;21(12):2061–8. https://doi.org/10.1016/j.bbmt.2015.08.030.
Jadus MR, Wepsic HT. The role of cytokines in graft-versus-host reactions and disease. Bone Marrow Transplant. 1992;10(1):1–14. Erratum in: Bone Marrow Transplant 1993 Jan;11(1):89.
Takatsuka H, Takemoto Y, Yamada S, Wada H, Tamura S, Fujimori Y, Okamoto T, Suehiro A, Kanamaru A, Kakishita E. Complications after bone marrow transplantation are manifestations of systemic inflammatory response syndrome. Bone Marrow Transplant. 2000;26(4):419–26. https://doi.org/10.1038/sj.bmt.1702517.
Khandelwal P, Mellor-Heineke S, Rehman N, Lane A, Smiley K, Villanueva J, Marsh RA, Grimley MS, Davies SM, Filipovich AH. Cytokine profile of engraftment syndrome in pediatric hematopoietic stem cell transplant recipients. Biol Blood Marrow Transplant. 2016;22(4):690–7. https://doi.org/10.1016/j.bbmt.2015.12.016.
Konuma T, Kohara C, Watanabe E, Mizukami M, Nagai E, Oiwa-Monna M, Tanoue S, Isobe M, Kato S, Tojo A, Takahashi S. Cytokine profiles of pre-engraftment syndrome after single-unit cord blood transplantation for adult patients. Biol Blood Marrow Transplant. 2017;23(11):1932–8. https://doi.org/10.1016/j.bbmt.2017.07.020.
Cornell RF, Hari P, Zhang MJ, Zhong X, Thompson J, Fenske TS, et al. Divergent effects of novel immunomodulatory agents and cyclophosphamide on the risk of engraftment syndrome after autologous peripheral blood stem cell transplantation for multiple myeloma. Biol Blood Marrow Transplant. 2013;19(9):1368–73. https://doi.org/10.1016/j.bbmt.2013.06.017;10.1016/j.bbmt.2013.06.017.
Sheth V, Jain R, Gore A, et al. Engraftment syndrome: clinical features and predictive factors in autologous stem cell transplant. Indian J Hematol Blood Transfus. 2018;34:448–53. https://doi.org/10.1007/s12288-017-0899-4.
Krenger W, Ferrara JL. Graft-versus-host disease and the Th1/Th2 paradigm. Immunol Res. 1996;15(1):50–73. https://doi.org/10.1007/BF02918284.
Nash RA, Antin JH, Karanes C, Fay JW, Avalos BR, Yeager AM, Przepiorka D, Davies S, Petersen FB, Bartels P, Buell D, Fitzsimmons W, Anasetti C, Storb R, Ratanatharathorn V. Phase 3 study comparing methotrexate and tacrolimus with methotrexate and cyclosporine for prophylaxis of acute graft-versus-host disease after marrow transplantation from unrelated donors. Blood. 2000;96(6):2062–8.
Ratanatharathorn V, Nash RA, Przepiorka D, Devine SM, Klein JL, Weisdorf D, Fay JW, Nademanee A, Antin JH, Christiansen NP, van der Jagt R, Herzig RH, Litzow MR, Wolff SN, Longo WL, Petersen FB, Karanes C, Avalos B, Storb R, Buell DN, Maher RM, Fitzsimmons WE, Wingard JR. Phase III study comparing methotrexate and tacrolimus (prograf, FK506) with methotrexate and cyclosporine for graft-versus-host disease prophylaxis after HLA-identical sibling bone marrow transplantation. Blood. 1998;92(7):2303–14.
Luznik L, O’Donnell PV, Symons HJ, Chen AR, Leffell MS, Zahurak M, Gooley TA, Piantadosi S, Kaup M, Ambinder RF, Huff CA, Matsui W, Bolaños-Meade J, Borrello I, Powell JD, Harrington E, Warnock S, Flowers M, Brodsky RA, Sandmaier BM, Storb RF, Jones RJ, Fuchs EJ. HLA-haploidentical bone marrow transplantation for hematologic malignancies using nonmyeloablative conditioning and high-dose, posttransplantation cyclophosphamide. Biol Blood Marrow Transplant. 2008;14(6):641–50. https://doi.org/10.1016/j.bbmt.2008.03.005.
Bacigalupo A, Lamparelli T, Bruzzi P, Guidi S, Alessandrino PE, di Bartolomeo P, Oneto R, Bruno B, Barbanti M, Sacchi N, Van Lint MT, Bosi A. Antithymocyte globulin for graft-versus-host disease prophylaxis in transplants from unrelated donors: 2 randomized studies from Gruppo Italiano Trapianti Midollo Osseo (GITMO). Blood. 2001;98(10):2942–7. https://doi.org/10.1182/blood.v98.10.2942.
Cutler C, Logan B, Nakamura R, Johnston L, Choi S, Porter D, Hogan WJ, Pasquini M, MacMillan ML, Hsu JW, Waller EK, Grupp S, McCarthy P, Wu J, Hu ZH, Carter SL, Horowitz MM, Antin JH. Tacrolimus/sirolimus vs tacrolimus/methotrexate as GVHD prophylaxis after matched, related donor allogeneic HCT. Blood. 2014;124(8):1372–7. https://doi.org/10.1182/blood-2014-04-567164.
Gooptu M, Antin JH. GVHD Prophylaxis 2020. Front Immunol. 2021;7(12):605726. https://doi.org/10.3389/fimmu.2021.605726.
Katzel JA, Mazumder A, Jagannath S, Vesole DH. Engraftment syndrome after hematopoietic stem cell transplantation in multiple myeloma. Clin Lymphoma Myeloma. 2006;7(2):151. https://doi.org/10.1016/S1557-9190(11)70311-0.
Gutiérrez-García G, Rovira M, Magnano L, Rosiñol L, Bataller A, Suárez-Lledó M, Cibeira MT, de Larrea CF, Garrote M, Jorge S, Moreno A, Rodríguez-Lobato LG, Carreras E, Díaz-Ricart M, Palomo M, Martínez C, Urbano-Ispizua A, Bladé J, Fernández-Avilés F. Innovative strategies minimize engraftment syndrome in multiple myeloma patients with novel induction therapy following autologous hematopoietic stem cell transplantation. Bone Marrow Transplant. 2018;53(12):1541–7. https://doi.org/10.1038/s41409-018-0189-2.
Irazabal MV, Eirin A, Gertz MA, Dispenzieri A, Kumar S, Buadi FK, Lacy MQ, Hayman SR, Dingli D, Hogan WJ, Gastineau DA, Glavey SV, Amer H, Leung N. Acute kidney injury during leukocyte engraftment after autologous stem cell transplantation in patients with light-chain amyloidosis. Am J Hematol. 2012;87(1):51–4. https://doi.org/10.1002/ajh.22202.
Carreras E, Fernández-Avilés F, Silva L, Guerrero M, de Fernández Larrea C, Martínez C, Rosiñol L, Lozano M, Marín P, Rovira M. Engraftment syndrome after auto-SCT: analysis of diagnostic criteria and risk factors in a large series from a single center. Bone Marrow Transplant. 2010;45(9):1417–22. https://doi.org/10.1038/bmt.2009.363.
Dispenzieri A, Lacy MQ, Hayman SR, Kumar SK, Buadi F, Dingli D, et al. Peripheral blood stem cell transplant for POEMS syndrome is associated with high rates of engraftment syndrome. Eur J Haematol. 2008;80(5):397–406. https://doi.org/10.1111/j.1600-0609.2008.01037.x.
Ileri T, Ünal Ince E, Çakmakli H, Uysal Z, Ertem M. Evaluation of engraftment syndrome in children following full matched related donor hematopoietic stem cell transplantations. Bone Marrow Transplant. 2016;51:S467–8. https://doi.org/10.1038/bmt.2016.49.
Omer AK, Kim HT, Yalamarti B, et al. Engraftment syndrome after allogeneic hematopoietic cell transplantation in adults. Am J Hematol. 2014;89(7):698–705. https://doi.org/10.1002/Ajh.23716.
Chang L, Frame D, Braun T, et al. Engraftment syndrome after allogeneic hematopoietic cell transplantation predicts poor outcomes. Biol Blood Marrow Transplant. 2014;20(9):1407–17. https://doi.org/10.1016/j.bbmt.2014.05.022.
Park M, Lee SH, Lee YH, et al. Pre-engraftment syndrome after unrelated cord blood transplantation: a predictor of engraftment and acute graft-versus-host disease. Biol Blood Marrow Transplant. 2013;19(4):640–6. https://doi.org/10.1016/j.bbmt.2013.01.014.
Wang X, Liu H, Li L, et al. Pre-engraftment syndrome after unrelated donor umbilical cord blood transplantation in patients with hematologic malignancies. Eur J Haematol. 2012;88(1):39–45. https://doi.org/10.1111/j.1600-0609.2011.01709.x.
Kanda J, Kaynar L, Kanda Y, et al. Pre-engraftment syndrome after myeloablative dual umbilical cord blood transplantation: risk factors and response to treatment. Bone Marrow Transplant. 2013;48(7):926–31. https://doi.org/10.1038/bmt.2012.279.
Acknowledgements
None.
Funding
Open access funding provided by Mid Sweden University. No funding was used from initiation till the completion of this project.
Author information
Authors and Affiliations
Contributions
SM: Conception, study design and literature search, MN: Conception, literature search and data extraction, AS: arrangement of literature and study design, KN, IK, AR, HT, KYL: Contributed in synthesis of systematic review, review and editing of the manuscript; RI, and FA: conception, design and supervision. All authors approved the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have declared that they have no competing interests.
Ethical approval
Not applicable.
Patients consent
Not Applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Maqbool, S., Nadeem, M., Shahroz, A. et al. Engraftment syndrome following Hematopoietic stem cell transplantation: a systematic approach toward diagnosis and management. Med Oncol 40, 36 (2023). https://doi.org/10.1007/s12032-022-01894-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12032-022-01894-7