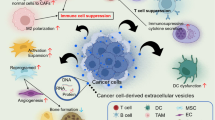
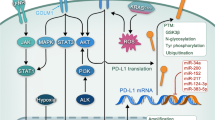
Abstract
Tumor cells exploit several mechanisms for hijacking an immunosuppressive tumor ecosystem in order to evade immune surveillance and to progress toward metastasis. Equipment of extracellular vesicles (EVs) with checkpoints is an example of cancer control over anti-tumor responses from immune system. Programmed death-ligand 1 (PD-L1) is a checkpoint highly expressed in a tumor at progressive stage. Interactions between PD-L1 with its receptor programmed death-1 receptor (PD-1) expressed on T cells will block the effector function of CD8+ T cells, known as one of the most important defensive cells against cancer. Evaluation of circulatory exosomal PD-L1 can be a prognostic biomarker in tumor diagnosis and responses to the immune checkpoint inhibitor (ICI) therapy, and can be considered as a tool in clinical practice for exploiting personalized therapy. Cytotoxic T lymphocyte-associated antigen-4 (CTLA-4) is also a checkpoint that its engagement with CD80/CD86 expressed on antigen-presenting cells (APCs), such as dendritic cells (DCs) hamper the priming phase of CD4+ and CD8+ T cells. Harvesting EVs from tumor and their modification with desired anti-checkpoint antibodies can be a promising strategy in cancer immunotherapy. The aim of this review is to discuss about EV roles in checkpoint regulation, cancer diagnosis and ICI responses, and to survey possible application of such vesicles in cancer immunotherapy.



Similar content being viewed by others
References
Majidpoor J, Mortezaee K. Angiogenesis as a hallmark of solid tumors-clinical perspectives. Cell Oncol. 2021. https://doi.org/10.1007/s13402-021-00602-3.
Majidpoor J, Mortezaee K. The efficacy of PD-1/PD-L1 blockade in cold cancers and future perspectives. Clin Immunol. 2021. https://doi.org/10.1016/j.clim.2021.108707.
Theodoraki M-N, Yerneni SS, Hoffmann TK, et al. Clinical significance of PD-L1+ exosomes in plasma of head and neck cancer patients. Clin Cancer Res. 2018;24(4):896–905.
Majidpoor J, Mortezaee K. Interleukin-6 in SARS-CoV-2 induced disease: interactions and therapeutic applications. Biomed Pharmacother. 2022;145: 112419.
Kim DH, Kim H, Choi YJ, et al. Exosomal PD-L1 promotes tumor growth through immune escape in non-small cell lung cancer. Exp Mol Med. 2019;51(8):1–13.
Rizvi NA, Hellmann MD, Snyder A, et al. Mutational landscape determines sensitivity to PD-1 blockade in non–small cell lung cancer. Science. 2015;348(6230):124–8.
Wang G, He L, Wang S, et al. EV PD-L1 is correlated with clinical features and contributes to T cell suppression in pediatric thyroid cancer. J Clin Endocrinol Metab. 2020;105(8):e2970–81.
Li L, Cao B, Liang X, et al. Microenvironmental oxygen pressure orchestrates an anti-and pro-tumoral γδ T cell equilibrium via tumor-derived exosomes. Oncogene. 2019;38(15):2830–43.
Poggio M, Hu T, Pai C-C, et al. Suppression of exosomal PD-L1 induces systemic anti-tumor immunity and memory. Cell. 2019;177(2):414-427.e13.
Pucci M, Raimondo S, Urzì O, et al. Tumor-derived small extracellular vesicles induce pro-inflammatory cytokine expression and PD-L1 regulation in M0 macrophages via IL-6/STAT3 and TLR4 signaling pathways. Int J Mol Sci. 2021;22(22):12118.
Zanella A, Vautrot V, Aubin F, et al. PD-L1 in circulating exosomes of Merkel cell carcinoma. Exp Dermatol. 2022. https://doi.org/10.1111/exd.14520.
Shimada Y, Matsubayashi J, Kudo Y, et al. Serum-derived exosomal PD-L1 expression to predict anti-PD-1 response and in patients with non-small cell lung cancer. Sci Rep. 2021;11(1):1–10.
Chen G, Huang AC, Zhang W, et al. Exosomal PD-L1 contributes to immunosuppression and is associated with anti-PD-1 response. Nature. 2018;560(7718):382–6.
Timaner M, Kotsofruk R, Raviv Z, et al. Microparticles from tumors exposed to radiation promote immune evasion in part by PD-L1. Oncogene. 2020;39(1):187–203.
Chen J, Lin Z, Liu L, et al. GOLM1 exacerbates CD8+ T cell suppression in hepatocellular carcinoma by promoting exosomal PD-L1 transport into tumor-associated macrophages. Signal Transduct Target Ther. 2021;6(1):1–15.
Mortezaee K, Majidpoor J. (Im) maturity in tumor ecosystem. Front Oncol. 2021;11:813897–813897.
Mortezaee K, Majidpoor J. Roles for macrophage-polarizing interleukins in cancer immunity and immunotherapy. Cell Oncol. 2022. https://doi.org/10.1007/s13402-022-00667-8.
Farhood B, Najafi M, Salehi E, et al. Disruption of the redox balance with either oxidative or anti-oxidative overloading as a promising target for cancer therapy. J Cell Biochem. 2019;120(1):71–6.
Mortezaee K. Hypoxia induces core-to-edge transition of progressive tumoral cells: a critical review on differential yet corroborative roles for HIF-1α and HIF-2α. Life Sci. 2020;242: 117145.
Mortezaee K, Majidpoor J. Checkpoint inhibitor/interleukin-based combination therapy of cancer. Cancer Med. 2022. https://doi.org/10.1002/cam4.4659.
Wang Y, Li P, Mao S, et al. Exosome CTLA-4 regulates PTEN/CD44 signal pathway in spleen deficiency internal environment to promote invasion and metastasis of hepatocellular carcinoma. Front Pharmacol. 2021. https://doi.org/10.3389/fphar.2021.757194.
Meldolesi J. Exosomes and ectosomes in intercellular communication. Curr Biol. 2018;28(8):R435–44.
Fan SJ, Kroeger B, Marie PP, et al. Glutamine deprivation alters the origin and function of cancer cell exosomes. EMBO J. 2020;39(16): e103009.
Najafi M, Majidpoor J, Toolee H, et al. The current knowledge concerning solid cancer and therapy. J Biochem Mol Toxicol. 2021. https://doi.org/10.1002/jbt.22900.
Mortezaee K. Redox tolerance and metabolic reprogramming in solid tumors. Cell Biol Int. 2021;45(2):273–86.
Tian W, Yang X, Yang H, et al. Exosomal miR-338-3p suppresses non-small-cell lung cancer cells metastasis by inhibiting CHL1 through the MAPK signaling pathway. Cell Death Dis. 2021;12(11):1–12.
Wen H, Liu Z, Tang J, et al. MiR-185-5p targets RAB35 gene to regulate tumor cell-derived exosomes-mediated proliferation, migration and invasion of non-small cell lung cancer cells. Aging (Albany NY). 2021;13(17):21435.
Wei L, Wang G, Yang C, et al. MicroRNA-550a-3-5p controls the brain metastasis of lung cancer by directly targeting YAP1. Cancer Cell Int. 2021;21(1):1–16.
Gao L, Nie X, Gou R, et al. Exosomal ANXA2 derived from ovarian cancer cells regulates epithelial-mesenchymal plasticity of human peritoneal mesothelial cells. J Cell Mol Med. 2021;25(23):10916–29.
Zhang C, Wang X-Y, Zhang P, et al. Cancer-derived exosomal HSPC111 promotes colorectal cancer liver metastasis by reprogramming lipid metabolism in cancer-associated fibroblasts. Cell Death Dis. 2022;13(1):1–14.
Shinde A, Paez JS, Libring S, et al. Transglutaminase-2 facilitates extracellular vesicle-mediated establishment of the metastatic niche. Oncogenesis. 2020;9(2):1–12.
Xie F, Zhou X, Fang M, et al. Extracellular vesicles in cancer immune microenvironment and cancer immunotherapy. Adv Sci. 2019;6(24):1901779.
Majidpoor J, Mortezaee K. Steps in metastasis: an updated review. Med Oncol. 2021;38(1):1–17.
Benito-Martin A, Di Giannatale A, Ceder S, et al. The new deal: a potential role for secreted vesicles in innate immunity and tumor progression. Front Immunol. 2015. https://doi.org/10.3389/fimmu.2015.00066.
Mortezaee K. Enriched cancer stem cells, dense stroma, and cold immunity: Interrelated events in pancreatic cancer. J Biochem Mol Toxicol. 2021;35(4): e22708.
Najafi M, Mortezaee K, Majidpoor J. Stromal reprogramming: a target for tumor therapy. Life Sci. 2019;239: 117049.
Su D, Tsai H-I, Xu Z, et al. Exosomal PD-L1 functions as an immunosuppressant to promote wound healing. J Extracell Vesicles. 2020;9(1):1709262.
Mortezaee K. Myeloid-derived suppressor cells in cancer immunotherapy-clinical perspectives. Life Sci. 2021;277: 119627.
Wang G, Xie L, Li B, et al. A nanounit strategy reverses immune suppression of exosomal PD-L1 and is associated with enhanced ferroptosis. Nat Commun. 2021;12(1):1–12.
Zhang L, Xue L, Wu Y, et al. Exosomes loaded with programmed death ligand-1 promote tumor growth by immunosuppression in osteosarcoma. Bioengineered. 2021;12(2):9520–30.
Yang Y, Li C-W, Chan L-C, et al. Exosomal PD-L1 harbors active defense function to suppress T cell killing of breast cancer cells and promote tumor growth. Cell Res. 2018;28(8):862–4.
Ricklefs FL, Alayo Q, Krenzlin H, et al. Immune evasion mediated by PD-L1 on glioblastoma-derived extracellular vesicles. Sci Adv. 2018;4(3):eaar2766.
Yang X, Zhang Y, Zhang Y, et al. The key role of exosomes on the pre-metastatic niche formation in tumors. Front Mol Biosci. 2021. https://doi.org/10.3389/fmolb.2021.703640.
Muller L, Simms P, Hong C-S, et al. Human tumor-derived exosomes (TEX) regulate Treg functions via cell surface signaling rather than uptake mechanisms. Oncoimmunology. 2017;6(8): e1261243.
Qiu Y, Yang Y, Yang R, et al. Activated T cell-derived exosomal PD-1 attenuates PD-L1-induced immune dysfunction in triple-negative breast cancer. Oncogene. 2021;40(31):4992–5001.
Fan Y, Che X, Qu J, et al. Exosomal PD-L1 retains immunosuppressive activity and is associated with gastric cancer prognosis. Ann Surg Oncol. 2019;26(11):3745–55.
Yeong J, Lim JCT, Lee B, et al. Prognostic value of CD8+ PD-1+ immune infiltrates and PDCD1 gene expression in triple negative breast cancer. J Immunother Cancer. 2019;7(1):1–13.
Li C, Qiu S, Jin K, et al. Tumor-derived microparticles promote the progression of triple-negative breast cancer via PD-L1-associated immune suppression. Cancer Lett. 2021;523:43–56.
Collier JL, Weiss SA, Pauken KE, et al. Not-so-opposite ends of the spectrum: CD8+ T cell dysfunction across chronic infection, cancer and autoimmunity. Nat Immunol. 2021;22(7):809–19.
Galletti G, De Simone G, Mazza E, et al. Two subsets of stem-like CD8+ memory T cell progenitors with distinct fate commitments in humans. Nat Immunol. 2020;21(12):1552–62.
Held W, Siddiqui I, Schaeuble K, et al. Intratumoral CD8+ T cells with stem cell–like properties: implications for cancer immunotherapy. Sci Transl Med. 2019;11(515):eaay6863.
Shin JM, Lee CH, Son S, et al. Sulfisoxazole elicits robust antitumour immune response along with immune checkpoint therapy by inhibiting exosomal PD-L1. Adv Sci. 2021. https://doi.org/10.1002/advs.202103245.
Wang X, Shen H, He Q, et al. Exosomes derived from exhausted CD8+ T cells impaired the anticancer function of normal CD8+ T cells. J Med Genet. 2019;56(1):29–31.
Chen J, Song Y, Miao F, et al. PDL1-positive exosomes suppress antitumor immunity by inducing tumor-specific CD8+ T cell exhaustion during metastasis. Cancer Sci. 2021;112(9):3437.
Zhao X, Yuan C, Wangmo D, et al. Tumor-secreted extracellular vesicles regulate T-cell costimulation and can be manipulated to induce tumor-specific T-cell responses. Gastroenterology. 2021;161(2):560-574.e11.
Ning Y, Shen K, Wu Q, et al. Tumor exosomes block dendritic cells maturation to decrease the T cell immune response. Immunol Lett. 2018;199:36–43.
Zhao Y, Liu L, Sun R, et al. Exosomes in cancer immunoediting and immunotherapy. Asian J Pharma Sci. 2022. https://doi.org/10.1016/j.ajps.2021.12.001.
Yan K, Da T-T, Bian Z-H, et al. Multi-omics analysis identifies FoxO1 as a regulator of macrophage function through metabolic reprogramming. Cell Death Dis. 2020;11(9):1–14.
Zhu Z, Zhang H, Chen B, et al. PD-L1-mediated immunosuppression in glioblastoma is associated with the infiltration and M2-polarization of tumor-associated macrophages. Front Immunol. 2020;11:2977.
Gabrusiewicz K, Li X, Wei J, et al. Glioblastoma stem cell-derived exosomes induce M2 macrophages and PD-L1 expression on human monocytes. Oncoimmunology. 2018;7(4): e1412909.
Liu J, Fan L, Yu H, et al. Endoplasmic reticulum stress causes liver cancer cells to release exosomal miR-23a-3p and up-regulate programmed death ligand 1 expression in macrophages. Hepatology. 2019;70(1):241–58.
Yao X, Tu Y, Xu Y, et al. Endoplasmic reticulum stress-induced exosomal miR-27a-3p promotes immune escape in breast cancer via regulating PD-L1 expression in macrophages. J Cell Mol Med. 2020;24(17):9560–73.
Zomer A, Maynard C, Verweij FJ, et al. In vivo imaging reveals extracellular vesicle-mediated phenocopying of metastatic behavior. Cell. 2015;161(5):1046–57.
Wu S, Sun R, Tan B, et al. The half-life-extended IL21 can be combined with multiple checkpoint inhibitors for tumor immunotherapy. Front Cell Dev Biol. 2021. https://doi.org/10.3389/fcell.2021.779865.
Cervera-Carrascon V, Quixabeira DC, Santos JM, et al. Adenovirus armed with TNFa and IL2 added to aPD-1 regimen mediates antitumor efficacy in tumors refractory to aPD-1. Front Immunol. 2021. https://doi.org/10.3389/fimmu.2021.706517.
Tucci M, Passarelli A, Mannavola F, et al. Serum exosomes as predictors of clinical response to ipilimumab in metastatic melanoma. Oncoimmunology. 2018;7(2): e1387706.
Hui E, Cheung J, Zhu J, et al. T cell costimulatory receptor CD28 is a primary target for PD-1–mediated inhibition. Science. 2017;355(6332):1428–33.
Del Re M, Marconcini R, Pasquini G, et al. PD-L1 mRNA expression in plasma-derived exosomes is associated with response to anti-PD-1 antibodies in melanoma and NSCLC. Br J Cancer. 2018;118(6):820–4.
Tamminga M, De Wit S, Hiltermann TJN, et al. Circulating tumor cells in advanced non-small cell lung cancer patients are associated with worse tumor response to checkpoint inhibitors. J Immunother Cancer. 2019;7(1):1–9.
Cordonnier M, Nardin C, Chanteloup G, et al. Tracking the evolution of circulating exosomal-PD-L1 to monitor melanoma patients. J Extracell Vesicles. 2020;9(1):1710899.
Hui R, Garon E, Goldman J, et al. Pembrolizumab as first-line therapy for patients with PD-L1-positive advanced non-small cell lung cancer: a phase 1 trial. Ann Oncol. 2017;28(4):874–81.
Aguiar PN Jr, De Mello RA, Hall P, et al. PD-L1 expression as a predictive biomarker in advanced non-small-cell lung cancer: updated survival data. Immunotherapy. 2017;9(6):499–506.
D’Angelo SP, Larkin J, Sosman JA, et al. Efficacy and safety of nivolumab alone or in combination with ipilimumab in patients with mucosal melanoma: a pooled analysis. J Clin Oncol. 2017;35(2):226.
Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med. 2015;373(1):23–34.
Lux A, Kahlert C, Grützmann R, et al. c-Met and PD-L1 on circulating exosomes as diagnostic and prognostic markers for pancreatic cancer. Int J Mol Sci. 2019;20(13):3305.
Li C, Li C, Zhi C, et al. Clinical significance of PD-L1 expression in serum-derived exosomes in NSCLC patients. J Transl Med. 2019;17(1):1–10.
McLaughlin J, Han G, Schalper KA, et al. Quantitative assessment of the heterogeneity of PD-L1 expression in non–small-cell lung cancer. JAMA Oncol. 2016;2(1):46–54.
Zhou J, Mahoney KM, Giobbie-Hurder A, et al. Soluble PD-L1 as a biomarker in malignant melanoma treated with checkpoint blockade. Cancer Immunol Res. 2017;5(6):480–92.
Kumagai S, Togashi Y, Kamada T, et al. The PD-1 expression balance between effector and regulatory T cells predicts the clinical efficacy of PD-1 blockade therapies. Nat Immunol. 2020;21(11):1346–58.
Gong B, Kiyotani K, Sakata S, et al. Secreted PD-L1 variants mediate resistance to PD-L1 blockade therapy in non–small cell lung cancer. J Exp Med. 2019;216(4):982–1000.
Dai Phung C, Pham TT, Nguyen HT, et al. Anti-CTLA-4 antibody-functionalized dendritic cell-derived exosomes targeting tumor-draining lymph nodes for effective induction of antitumor T-cell responses. Acta Biomater. 2020;115:371–82.
Shi S, Rao Q, Zhang C, et al. Dendritic cells pulsed with exosomes in combination with PD-1 antibody increase the efficacy of sorafenib in hepatocellular carcinoma model. Trans Oncol. 2018;11(2):250–8.
Costa-Silva B, Aiello NM, Ocean AJ, et al. Pancreatic cancer exosomes initiate pre-metastatic niche formation in the liver. Nat Cell Biol. 2015;17(6):816–26.
Peinado H, Alečković M, Lavotshkin S, et al. Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET. Nat Med. 2012;18(6):883–91.
Kaplan RN, Riba RD, Zacharoulis S, et al. VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature. 2005;438(7069):820–7.
Li C-W, Lim S-O, Xia W, et al. Glycosylation and stabilization of programmed death ligand-1 suppresses T-cell activity. Nat Commun. 2016;7(1):1–11.
Acknowledgements
This work is supported by the approval from Ethical Committee of Kurdistan University of Medical Sciences (The ethical code: IR.MUK.REC.1401.112).
Funding
No funding is received for this paper.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declares that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mortezaee, K., Majidpoor, J. Extracellular vesicle-based checkpoint regulation and immune state in cancer. Med Oncol 39, 225 (2022). https://doi.org/10.1007/s12032-022-01837-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12032-022-01837-2