Abstract
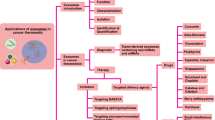
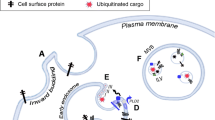
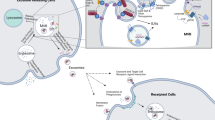
Exosomes are extracellular nanovesicles secreted from almost all types of normal and cancer cells. Collective evidence suggests that exosomes participate in cell–cell communication via transmitting their cargo, including nucleic acids, proteins, and metabolites to recipient cells. Tumor-derived exosomes (TEXs) play prominent roles in the regulation of molecular pathways in malignancies. Internalization of exosomes by tumor cells affects cellular pathways and several cancer hallmarks, including reprogramming of stromal cells, modulating immune responses, reconstructing extracellular matrix architecture, or even endowing tumor cells with drug features resistance. The unique biogenesis pathways of exosomes, their composition, low immunogenicity, and nontoxicity, together with their ability to target tumor cells, bring them up as an attractive vesicles for cancer therapy. Thus, understanding the molecular mechanisms of exosomes' participation in tumorigenesis will be critical for the next generation of cancer therapeutics. This review aims to summarize the exosomes' roles in different mechanisms underlying cancer progression for the rational design of tailored strategies against this illness. The present study also highlights the new findings on using these smart vesicles as therapeutic targets and potential biomarkers. Recent advances in exosome biology will open up new, more effective, less invasive, and more individualized clinical applications for treating cancer patients.



Similar content being viewed by others
Abbreviations
- ABCs:
-
ATP-binding cassette transporters
- AD:
-
Alzheimer's disease
- Aβ:
-
β-Amyloid
- bFGF:
-
Basic fibroblast growth factor
- CNS:
-
Central nervous system
- CSF:
-
Cerebrospinal fluid
- DCs:
-
Dendritic cells
- EMT:
-
Epithelial to mesenchymal transition
- ERK1/2:
-
Extracellular signal‑regulated protein kinase
- EVs:
-
Extracellular vesicles
- FAK:
-
Focal adhesion kinase
- HSP:
-
Heat shock protein
- IFN:
-
Interferon
- IL:
-
Interleukin
- MAPK:
-
Mitogen-activated protein kinase
- MHC:
-
Major histocompatibility complex
- MMP:
-
Matrix metalloproteinase
- MSCs:
-
Mesenchymal stem cells
- MVBs:
-
Multivesicular bodies
- NF-κB:
-
Nuclear factor κB
- NOS:
-
Nitric oxide synthase
- NSCLC:
-
Non-small cell lung cancer
- PD-1:
-
Programmed death 1
- PDGF:
-
Platelet-derived growth factor
- P-gp:
-
P-glycoprotein
- PI3K/Akt:
-
Phosphoinositide 3-kinase/protein kinase B
- TERT:
-
Telomerase reverse transcriptase
- TEXs:
-
Tumor-derived exosomes
- TGF-β:
-
Transforming growth factor β
- TME:
-
Tumor microenvironment
- VEGF:
-
Vascular endothelial growth factor
References
Jafari A, Rezaei-Tavirani M, Farhadihosseinabadi B, Zali H, Niknejad H. Human amniotic mesenchymal stem cells to promote/suppress cancer: two sides of the same coin. Stem Cell Res Ther. 2021;12:126. https://doi.org/10.1186/s13287-021-02196-x.
Babajani A, Soltani P, Jamshidi E, Farjoo MH, Niknejad H. Recent advances on drug-loaded mesenchymal stem cells with anti-neoplastic agents for targeted treatment of cancer. Front Bioeng Biotechnol. 2020;8:748. https://doi.org/10.3389/fbioe.2020.00748.
Dai J, Su Y, Zhong S, Cong L, Liu B, Yang J, et al. Exosomes: key players in cancer and potential therapeutic strategy. Signal Transduct Target Ther. 2020;5:145. https://doi.org/10.1038/s41392-020-00261-0.
Johnstone RM, Adam M, Hammond JR, Orr L, Turbide C. Vesicle formation during reticulocyte maturation. Association of plasma membrane activities with released vesicles (exosomes). J Biol Chem. 1987;262:9412–20.
Vidal M. Exosomes: revisiting their role as “garbage bags.” Traffic. 2019;20:815–28. https://doi.org/10.1111/tra.12687.
Wu R, Gao W, Yao K, Ge J. Roles of exosomes derived from immune cells in cardiovascular diseases. Front Immunol. 2019;10:648. https://doi.org/10.3389/fimmu.2019.00648.
Jamshidi E, Babajani A, Soltani P, Niknejad H. Proposed mechanisms of targeting COVID-19 by delivering mesenchymal stem cells and their exosomes to damaged organs. Stem Cell Rev Rep. 2021. https://doi.org/10.1007/s12015-020-10109-3.
Barceló M, Castells M, Bassas L, Vigués F, Larriba S. Semen miRNAs contained in exosomes as non-invasive biomarkers for prostate cancer diagnosis. Sci Rep. 2019;9:13772. https://doi.org/10.1038/s41598-019-50172-6.
Sagredo AI, Sepulveda SA, Roa JC, Oróstica L. Exosomes in bile as potential pancreatobiliary tumor biomarkers. Trans Cancer Res. 2017. https://doi.org/10.21037/tcr.2017.10.37.
Kumar SR, Kimchi ET, Manjunath Y, Gajagowni S, Stuckel AJ, Kaifi JT. RNA cargos in extracellular vesicles derived from blood serum in pancreas associated conditions. Sci Rep. 2020;10:2800. https://doi.org/10.1038/s41598-020-59523-0.
Mirza AH, Kaur S, Nielsen LB, Størling J, Yarani R, Roursgaard M, et al. Breast milk-derived extracellular vesicles enriched in exosomes from mothers with type 1 diabetes contain aberrant levels of microRNAs. Front Immunol. 2019;10:2543. https://doi.org/10.3389/fimmu.2019.02543.
Pisitkun T, Shen R-F, Knepper MA. Identification and proteomic profiling of exosomes in human urine. Proc Natl Acad Sci USA. 2004. https://doi.org/10.1073/pnas.0403453101.
Michael A, Bajracharya SD, Yuen PST, Zhou H, Star RA, Illei GG, et al. Exosomes from human saliva as a source of microRNA biomarkers. Oral Dis. 2010;16:34–8. https://doi.org/10.1111/j.1601-0825.2009.01604.x.
Capello M, Vykoukal JV, Katayama H, Bantis LE, Wang H, Kundnani DL, et al. Exosomes harbor B cell targets in pancreatic adenocarcinoma and exert decoy function against complement-mediated cytotoxicity. Nat Commun. 2019;10:254. https://doi.org/10.1038/s41467-018-08109-6.
Ogorevc E, Kralj-Iglic V, Veranic P. The role of extracellular vesicles in phenotypic cancer transformation. Radiol Oncol. 2013. https://doi.org/10.2478/raon-2013-0037.
Zhang W, Zhou Q, Wei Y, Da M, Zhang C, Zhong J, et al. The exosome-mediated PI3k/Akt/mTOR signaling pathway in cervical cancer. Int J Clin Exp Pathol. 2019;12:2474–84.
Zhang Y, Liu F, Yuan Y, Jin C, Chang C, Zhu Y, et al. Inflammasome-derived exosomes activate NF-κB signaling in macrophages. J Proteome Res. 2017;16:170–8. https://doi.org/10.1021/acs.jproteome.6b00599.
Liu Z, Wu C, Zou X, Shen W, Yang J, Zhang X, et al. Exosomes derived from mesenchymal stem cells inhibit neointimal hyperplasia by activating the Erk1/2 signalling pathway in rats. Stem Cell Res Ther. 2020;11:220. https://doi.org/10.1186/s13287-020-01676-w.
Dovrat S, Caspi M, Zilberberg A, Lahav L, Firsow A, Gur H, et al. 14–3-3 and β-catenin are secreted on extracellular vesicles to activate the oncogenic Wnt pathway. Mol Oncol. 2014;8:894–911. https://doi.org/10.1016/j.molonc.2014.03.011.
Shelke GV, Yin Y, Jang SC, Lässer C, Wennmalm S, Hoffmann HJ, et al. Endosomal signalling via exosome surface TGFβ-1. J Extracell Vesicles. 2019. https://doi.org/10.1080/20013078.2019.1650458.
Wang Q, Lu Q. Plasma membrane-derived extracellular microvesicles mediate non-canonical intercellular NOTCH signaling. Nat Commun. 2017;8:709. https://doi.org/10.1038/s41467-017-00767-2.
Lai RC, Arslan F, Lee MM, Sze NSK, Choo A, Chen TS, et al. Exosome secreted by MSC reduces myocardial ischemia/reperfusion injury. Stem Cell Res. 2010;4:214–22. https://doi.org/10.1016/j.scr.2009.12.003.
Liu W, Bai X, Zhang A, Huang J, Xu S, Zhang J. Role of exosomes in central nervous system diseases. Front Mol Neurosci. 2019;12:240. https://doi.org/10.3389/fnmol.2019.00240.
Zhang S, Hou Y, Yang J, Xie D, Jiang L, Hu H, et al. Application of mesenchymal stem cell exosomes and their drug-loading systems in acute liver failure. J Cell Mol Med. 2020;24:7082–93. https://doi.org/10.1111/jcmm.15290.
Wang B, Yao K, Huuskes BM, Shen H-H, Zhuang J, Godson C, et al. Mesenchymal stem cells deliver exogenous MicroRNA-let7c via exosomes to attenuate renal fibrosis. Mol Ther. 2016;24:1290–301. https://doi.org/10.1038/mt.2016.90.
Ye S-B, Li Z-L, Luo D-H, Huang B-J, Chen Y-S, Zhang X-S, et al. Tumor-derived exosomes promote tumor progression and T-cell dysfunction through the regulation of enriched exosomal microRNAs in human nasopharyngeal carcinoma. Oncotarget. 2014;5:5439–52. https://doi.org/10.18632/oncotarget.2118.
Mittelbrunn M, Sánchez-Madrid F. Intercellular communication: diverse structures for exchange of genetic information. Nat Rev Mol Cell Biol. 2012;13:328–35. https://doi.org/10.1038/nrm3335.
Bellingham SA, Guo BB, Coleman BM, Hill AF. Exosomes: vehicles for the transfer of toxic proteins associated with neurodegenerative diseases? Front Physiol. 2012;3:124. https://doi.org/10.3389/fphys.2012.00124.
Jung MK, Mun JY. Sample preparation and imaging of exosomes by transmission electron microscopy. J Vis Exp. 2018. https://doi.org/10.3791/56482.
Luan X, Sansanaphongpricha K, Myers I, Chen H, Yuan H, Sun D. Engineering exosomes as refined biological nanoplatforms for drug delivery. Acta Pharmacol Sin. 2017. https://doi.org/10.1038/aps.2017.12.
Mathivanan S, Ji H, Simpson RJ. Exosomes: extracellular organelles important in intercellular communication. J Proteomics. 2010. https://doi.org/10.1016/j.jprot.2010.06.006.
Llorente A, Skotland T, Sylvänne T, Kauhanen D, Róg T, Orłowski A, et al. Molecular lipidomics of exosomes released by PC-3 prostate cancer cells. Biochim Biophys Acta. 2013;1831:1302–9. https://doi.org/10.1016/j.bbalip.2013.04.011.
Escrevente C, Grammel N, Kandzia S, Zeiser J, Tranfield EM, Conradt HS, et al. Sialoglycoproteins and N-glycans from secreted exosomes of ovarian carcinoma cells. PLoS ONE. 2013;8:e78631. https://doi.org/10.1371/journal.pone.0078631.
Yoshioka Y, Konishi Y, Kosaka N, Katsuda T, Kato T, Ochiya T. Comparative marker analysis of extracellular vesicles in different human cancer types. J Extracell Vesicles. 2013. https://doi.org/10.3402/jev.v2i0.20424.
Hoshino A, Costa-Silva B, Shen T-L, Rodrigues G, Hashimoto A, Tesic Mark M, et al. Tumour exosome integrins determine organotropic metastasis. Nature. 2015;527:329–35. https://doi.org/10.1038/nature15756.
Samsonov R, Shtam T, Burdakov V, Glotov A, Tsyrlina E, Berstein L, et al. Lectin-induced agglutination method of urinary exosomes isolation followed by mi-RNA analysis: application for prostate cancer diagnostic. Prostate. 2016;76:68–79. https://doi.org/10.1002/pros.23101.
Urbanelli L, Magini A, Buratta S, Brozzi A, Sagini K, Polchi A, et al. Signaling pathways in exosomes biogenesis, secretion and fate. Genes. 2013;4:152–70. https://doi.org/10.3390/genes4020152.
Baaklini I, de Gonçalves C, Lukacs GL, Young JC. Selective binding of HSC70 and its co-chaperones to structural hotspots on CFTR. Sci Rep. 2020;10:4176. https://doi.org/10.1038/s41598-020-61107-x.
Simpson RJ, Lim JW, Moritz RL, Mathivanan S. Exosomes: proteomic insights and diagnostic potential. Expert Rev Proteomics. 2009;6:267–83. https://doi.org/10.1586/epr.09.17.
Lian Q, Xu J, Yan S, Huang M, Ding H, Sun X, et al. Chemotherapy-induced intestinal inflammatory responses are mediated by exosome secretion of double-strand DNA via AIM2 inflammasome activation. Cell Res. 2017;27:784–800. https://doi.org/10.1038/cr.2017.54.
Sharma A, Johnson A. Exosome DNA: critical regulator of tumor immunity and a diagnostic biomarker. J Cell Physiol. 2020. https://doi.org/10.1002/jcp.29153.
Keerthikumar S, Chisanga D, Ariyaratne D, Al Saffar H, Anand S, Zhao K, et al. ExoCarta: a web-based compendium of exosomal Cargo. J Mol Biol. 2016;428:688–92. https://doi.org/10.1016/j.jmb.2015.09.019.
Anand PK, Anand E, Bleck CKE, Anes E, Griffiths G. Exosomal Hsp70 induces a pro-inflammatory response to foreign particles including mycobacteria. PLoS ONE. 2010;5:e10136. https://doi.org/10.1371/journal.pone.0010136.
Quek C, Hill AF. The role of extracellular vesicles in neurodegenerative diseases. Biochem Biophys Res Commun. 2017;483:1178–86. https://doi.org/10.1016/j.bbrc.2016.09.090.
Lim CZJ, Zhang Y, Chen Y, Zhao H, Stephenson MC, Ho NRY, et al. Subtyping of circulating exosome-bound amyloid β reflects brain plaque deposition. Nat Commun. 2019;10:1144. https://doi.org/10.1038/s41467-019-09030-2.
Rajendran L, Honsho M, Zahn TR, Keller P, Geiger KD, Verkade P, et al. Alzheimer’s disease beta-amyloid peptides are released in association with exosomes. Proc Natl Acad Sci U S A. 2006;103:11172–7. https://doi.org/10.1073/pnas.0603838103.
Wang Y, Xu F, Zhong J-Y, Lin X, Shan S-K, Guo B, et al. Exosomes as mediators of cell-to-cell communication in thyroid disease. Int J Endocrinol. 2020;2020:4378345. https://doi.org/10.1155/2020/4378345.
Liu H, Chen L, Peng Y, Yu S, Liu J, Wu L, et al. Dendritic cells loaded with tumor derived exosomes for cancer immunotherapy. Oncotarget. 2018;9:2887–94. https://doi.org/10.18632/oncotarget.20812.
Zhou Y, Tian T, Zhu Y, Jaffar Ali D, Hu F, Qi Y, et al. Exosomes transfer among different species cells and mediating miRNAs delivery. J Cell Biochem. 2017;118:4267–74. https://doi.org/10.1002/jcb.26077.
Zhang W, Zhou X, Yao Q, Liu Y, Zhang H, Dong Z. HIF-1-mediated production of exosomes during hypoxia is protective in renal tubular cells. Am J Physiol Renal Physiol. 2017;313:F906–13. https://doi.org/10.1152/ajprenal.00178.2017.
Wang J, De Veirman K, Faict S, Frassanito MA, Ribatti D, Vacca A, et al. Multiple myeloma exosomes establish a favourable bone marrow microenvironment with enhanced angiogenesis and immunosuppression. J Pathol. 2016;239:162–73. https://doi.org/10.1002/path.4712.
Kucharzewska P, Christianson HC, Welch JE, Svensson KJ, Fredlund E, Ringnér M, et al. Exosomes reflect the hypoxic status of glioma cells and mediate hypoxia-dependent activation of vascular cells during tumor development. Proc Natl Acad Sci U S A. 2013;110:7312–7. https://doi.org/10.1073/pnas.1220998110.
Webber J, Steadman R, Mason MD, Tabi Z, Clayton A. Cancer exosomes trigger fibroblast to myofibroblast differentiation. Cancer Res. 2010;70:9621–30. https://doi.org/10.1158/0008-5472.CAN-10-1722.
Nazarenko I, Rana S, Baumann A, McAlear J, Hellwig A, Trendelenburg M, et al. Cell surface tetraspanin Tspan8 contributes to molecular pathways of exosome-induced endothelial cell activation. Cancer Res. 2010;70:1668–78. https://doi.org/10.1158/0008-5472.CAN-09-2470.
Yang W-J, Yan J-B, Zhang L, Zhao F, Mei Z-M, Yang Y-N, et al. Paxillin promotes the migration and angiogenesis of HUVECs and affects angiogenesis in the mouse cornea. Exp Ther Med. 2020;20:901–9. https://doi.org/10.3892/etm.2020.8751.
Bryan BA, Dennstedt E, Mitchell DC, Walshe TE, Noma K, Loureiro R, et al. RhoA/ROCK signaling is essential for multiple aspects of VEGF-mediated angiogenesis. FASEB J. 2010;24:3186–95. https://doi.org/10.1096/fj.09-145102.
Weddell JC, Chen S, Imoukhuede PI. VEGFR1 promotes cell migration and proliferation through PLCγ and PI3K pathways. NPJ Syst Biol Appl. 2018;4:1. https://doi.org/10.1038/s41540-017-0037-9.
DeRita RM, Zerlanko B, Singh A, Lu H, Iozzo RV, Benovic JL, et al. c-Src, insulin-like growth factor I receptor, G-protein-coupled receptor kinases and focal adhesion kinase are enriched into prostate cancer cell exosomes. J Cell Biochem. 2017;118:66–73. https://doi.org/10.1002/jcb.25611.
Guo P, Hu B, Gu W, Xu L, Wang D, Huang H-JS, et al. Platelet-derived growth factor-B enhances glioma angiogenesis by stimulating vascular endothelial growth factor expression in tumor endothelia and by promoting pericyte recruitment. Am J Pathol. 2003;162:1083–93. https://doi.org/10.1016/S0002-9440(10)63905-3.
Webb AH, Gao BT, Goldsmith ZK, Irvine AS, Saleh N, Lee RP, et al. Inhibition of MMP-2 and MMP-9 decreases cellular migration and angiogenesis in in vitro models of retinoblastoma. BMC Cancer. 2017. https://doi.org/10.1186/s12885-017-3418-y.
Sun B, Zhou Y, Fang Y, Li Z, Gu X, Xiang J. Colorectal cancer exosomes induce lymphatic network remodeling in lymph nodes. Int J Cancer. 2019;145:1648–59. https://doi.org/10.1002/ijc.32196.
Li R, Wang Y, Zhang X, Feng M, Ma J, Li J, et al. Exosome-mediated secretion of LOXL4 promotes hepatocellular carcinoma cell invasion and metastasis. Mol Cancer. 2019;18:18. https://doi.org/10.1186/s12943-019-0948-8.
Somasundaram R, Herlyn M. Melanoma exosomes: messengers of metastasis. Nat Med. 2012. https://doi.org/10.1038/nm.2775.
Zhou W, Fong MY, Min Y, Somlo G, Liu L, Palomares MR, et al. Cancer-secreted miR-105 destroys vascular endothelial barriers to promote metastasis. Cancer Cell. 2014;25:501–15. https://doi.org/10.1016/j.ccr.2014.03.007.
Tominaga N, Kosaka N, Ono M, Katsuda T, Yoshioka Y, Tamura K, et al. Brain metastatic cancer cells release microRNA-181c-containing extracellular vesicles capable of destructing blood-brain barrier. Nat Commun. 2015;6:6716. https://doi.org/10.1038/ncomms7716.
McCready J, Sims JD, Chan D, Jay DG. Secretion of extracellular hsp90α via exosomes increases cancer cell motility: a role for plasminogen activation. BMC Cancer. 2010. https://doi.org/10.1186/1471-2407-10-294.
Peinado H, Alečković M, Lavotshkin S, Matei I, Costa-Silva B, Moreno-Bueno G, et al. Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET. Nat Med. 2012;18:883–91. https://doi.org/10.1038/nm.2753.
Hendrix A, Braems G, Bracke M, Seabra M, Gahl W, De Wever O, et al. The secretory small GTPase Rab27B as a marker for breast cancer progression. Oncotarget. 2010;1:304–8. https://doi.org/10.18632/oncotarget.100809.
Hoshino D, Kirkbride KC, Costello K, Clark ES, Sinha S, Grega-Larson N, et al. Exosome secretion is enhanced by invadopodia and drives invasive behavior. Cell Rep. 2013;5:1159–68. https://doi.org/10.1016/j.celrep.2013.10.050.
Eddy RJ, Weidmann MD, Sharma VP, Condeelis JS. Tumor Cell invadopodia: invasive protrusions that orchestrate metastasis. Trends Cell Biol. 2017;27:595–607. https://doi.org/10.1016/j.tcb.2017.03.003.
Bobrie A, Krumeich S, Reyal F, Recchi C, Moita LF, Seabra MC, et al. Rab27a supports exosome-dependent and -independent mechanisms that modify the tumor microenvironment and can promote tumor progression. Cancer Res. 2012;72:4920–30. https://doi.org/10.1158/0008-5472.CAN-12-0925.
Liu Y, Gu Y, Han Y, Zhang Q, Jiang Z, Zhang X, et al. Tumor Exosomal RNAs promote lung pre-metastatic niche formation by activating alveolar epithelial TLR3 to recruit neutrophils. Cancer Cell. 2016;30:243–56. https://doi.org/10.1016/j.ccell.2016.06.021.
Zhang X, Shi H, Yuan X, Jiang P, Qian H, Xu W. Tumor-derived exosomes induce N2 polarization of neutrophils to promote gastric cancer cell migration. Mol Cancer. 2018;17:146. https://doi.org/10.1186/s12943-018-0898-6.
Wang S, Su X, Xu M, Xiao X, Li X, Li H, et al. Exosomes secreted by mesenchymal stromal/stem cell-derived adipocytes promote breast cancer cell growth via activation of Hippo signaling pathway. Stem Cell Res Ther. 2019;10:117. https://doi.org/10.1186/s13287-019-1220-2.
Li Z, Wang Y, Zhu Y, Yuan C, Wang D, Zhang W, et al. The Hippo transducer TAZ promotes epithelial to mesenchymal transition and cancer stem cell maintenance in oral cancer. Mol Oncol. 2015. https://doi.org/10.1016/j.molonc.2015.01.007.
Hu JL, Wang W, Lan XL, Zeng ZC, Liang YS, Yan YR, et al. CAFs secreted exosomes promote metastasis and chemotherapy resistance by enhancing cell stemness and epithelial-mesenchymal transition in colorectal cancer. Mol Cancer. 2019;18:91. https://doi.org/10.1186/s12943-019-1019-x.
Han M, Liu M, Wang Y, Chen X, Xu J, Sun Y, et al. Antagonism of miR-21 Reverses Epithelial-Mesenchymal Transition And Cancer Stem Cell Phenotype Through AKT/ERK1/2 inactivation by targeting PTEN. PLoS ONE. 2012. https://doi.org/10.1371/journal.pone.0039520.
Ying X, Wu Q, Wu X, Zhu Q, Wang X, Jiang L, et al. Epithelial ovarian cancer-secreted exosomal miR-222–3p induces polarization of tumor-associated macrophages. Oncotarget. 2016. https://doi.org/10.18632/oncotarget.9246.
Ding J, Xu Z, Zhang Y, Tan C, Hu W, Wang M, et al. Exosome-mediated miR-222 transferring: an insight into NF-κB-mediated breast cancer metastasis. Exp Cell Res. 2018. https://doi.org/10.1016/j.yexcr.2018.05.014.
Zhang L, Zhang S, Yao J, Lowery FJ, Zhang Q, Huang W-C, et al. Microenvironment-induced PTEN loss by exosomal microRNA primes brain metastasis outgrowth. Nature. 2015. https://doi.org/10.1038/nature15376.
Lin R, Wang S, Zhao RC. Exosomes from human adipose-derived mesenchymal stem cells promote migration through Wnt signaling pathway in a breast cancer cell model. Mol Cell Biochem. 2013. https://doi.org/10.1007/s11010-013-1746-z.
Sánchez CA, Andahur EI, Valenzuela R, Castellón EA, Fullá JA, Ramos CG, et al. Exosomes from bulk and stem cells from human prostate cancer have a differential microRNA content that contributes cooperatively over local and pre-metastatic niche. Oncotarget. 2016. https://doi.org/10.18632/oncotarget.6540.
Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324:1029–33. https://doi.org/10.1126/science.1160809.
Ward C, Meehan J, Gray ME, Murray AF, Argyle DJ, Kunkler IH, et al. The impact of tumour pH on cancer progression: strategies for clinical intervention. Explor Target Anti-tumor Ther. 2020;1:71–100. https://doi.org/10.37349/etat.2020.00005.
Fischer K, Hoffmann P, Voelkl S, Meidenbauer N, Ammer J, Edinger M, et al. Inhibitory effect of tumor cell–derived lactic acid on human T cells. Blood. 2007. https://doi.org/10.1182/blood-2006-07-035972.
Husain Z, Huang Y, Seth P, Sukhatme VP. Tumor-derived lactate modifies antitumor immune response: effect on myeloid-derived suppressor cells and NK cells. J Immunol. 2013. https://doi.org/10.4049/jimmunol.1202702.
Parolini I, Federici C, Raggi C, Lugini L, Palleschi S, De Milito A, et al. Microenvironmental pH is a key factor for exosome traffic in tumor cells. J Biol Chem. 2009;284:34211–22. https://doi.org/10.1074/jbc.M109.041152.
Zhao H, Yang L, Baddour J, Achreja A, Bernard V, Moss T, et al. Tumor microenvironment derived exosomes pleiotropically modulate cancer cell metabolism. Elife. 2016;5:e10250. https://doi.org/10.7554/eLife.10250.
Shu SL, Yang Y, Allen CL, Maguire O, Minderman H, Sen A, et al. Metabolic reprogramming of stromal fibroblasts by melanoma exosome microRNA favours a pre-metastatic microenvironment. Sci Rep. 2018;8:12905. https://doi.org/10.1038/s41598-018-31323-7.
Shephard AP, Yeung V, Clayton A, Webber JP. Prostate cancer exosomes as modulators of the tumor microenvironment. J Cancer Metastas Treat. 2017. https://doi.org/10.20517/2394-4722.2017.32.
Ronquist KG, Göran Ronquist K, Ek B, Morrell J, Stavreus-Evers A, Holst BS, et al. Prostasomes from four different species are able to produce extracellular adenosine triphosphate (ATP). Biochim Biophys Acta (BBA). 2013. https://doi.org/10.1016/j.bbagen.2013.05.019.
Clayton A, Al-Taei S, Webber J, Mason MD, Tabi Z. Cancer exosomes express CD39 and CD73, which suppress T cells through adenosine production. J Immunol. 2011;187:676–83. https://doi.org/10.4049/jimmunol.1003884.
Escrevente C, Keller S, Altevogt P, Costa J. Interaction and uptake of exosomes by ovarian cancer cells. BMC Cancer. 2011;11:108. https://doi.org/10.1186/1471-2407-11-108.
Qian Y, Wang X, Li Y, Cao Y, Chen X. Abstract 33: NSCLC cells internalize ATPin vitroandin vivousing multiple endocytotic pathways. Mol Cell Biol Genet. 2016. https://doi.org/10.1158/1538-7445.am2016-33.
Carneiro BA, El-Deiry WS. Targeting apoptosis in cancer therapy. Nat Rev Clin Oncol. 2020;17:395–417. https://doi.org/10.1038/s41571-020-0341-y.
Qu J-L, Qu X-J, Zhao M-F, Teng Y-E, Zhang Y, Hou K-Z, et al. Gastric cancer exosomes promote tumour cell proliferation through PI3K/Akt and MAPK/ERK activation. Dig Liver Dis. 2009;41:875–80. https://doi.org/10.1016/j.dld.2009.04.006.
Li C, Liu D-R, Li G-G, Wang H-H, Li X-W, Zhang W, et al. CD97 promotes gastric cancer cell proliferation and invasion through exosome-mediated MAPK signaling pathway. World J Gastroenterol. 2015;21:6215–28. https://doi.org/10.3748/wjg.v21.i20.6215.
Yang L, Wu X-H, Wang D, Luo C-L, Chen L-X. Bladder cancer cell-derived exosomes inhibit tumor cell apoptosis and induce cell proliferation in vitro. Mol Med Rep. 2013;8:1272–8. https://doi.org/10.3892/mmr.2013.1634.
Huang J, Ding Z, Luo Q, Xu W. Cancer cell-derived exosomes promote cell proliferation and inhibit cell apoptosis of both normal lung fibroblasts and non-small cell lung cancer cell through delivering alpha-smooth muscle actin. Am J Transl Res. 2019;11:1711–23.
Dave B, Granados-Principal S, Zhu R, Benz S, Rabizadeh S, Soon-Shiong P, et al. Targeting RPL39 and MLF2 reduces tumor initiation and metastasis in breast cancer by inhibiting nitric oxide synthase signaling. Proc Natl Acad Sci U S A. 2014;111:8838–43. https://doi.org/10.1073/pnas.1320769111.
ExoCarta: Home - Exosome database. http://www.exocarta.org/ (2020). Accessed 18 Nov 2020
Fernandes JV, Cobucci RNO, Jatobá CAN, de Fernandes TAA, de Azevedo JWV, de Araújo JMG. The role of the mediators of inflammation in cancer development. Pathol Oncol Res. 2015;21:527–34. https://doi.org/10.1007/s12253-015-9913-z.
Sharma P, Diergaarde B, Ferrone S, Kirkwood JM, Whiteside TL. Melanoma cell-derived exosomes in plasma of melanoma patients suppress functions of immune effector cells. Sci Rep. 2020;10:92. https://doi.org/10.1038/s41598-019-56542-4.
Shen Y, Guo D, Weng L, Wang S, Ma Z, Yang Y, et al. Tumor-derived exosomes educate dendritic cells to promote tumor metastasis via HSP72/HSP105-TLR2/TLR4 pathway. Oncoimmunology. 2017;6:e1362527. https://doi.org/10.1080/2162402X.2017.1362527.
Wang X, Luo G, Zhang K, Cao J, Huang C, Jiang T, et al. Hypoxic tumor-derived exosomal miR-301a mediates M2 macrophage polarization via PTEN/PI3Kγ to promote pancreatic cancer metastasis. Cancer Res. 2018;78:4586–98. https://doi.org/10.1158/0008-5472.CAN-17-3841.
Blank C, Gajewski TF, Mackensen A. Interaction of PD-L1 on tumor cells with PD-1 on tumor-specific T cells as a mechanism of immune evasion: implications for tumor immunotherapy. Cancer Immunol Immunother. 2005;54:307–14. https://doi.org/10.1007/s00262-004-0593-x.
Chen G, Huang AC, Zhang W, Zhang G, Wu M, Xu W, et al. Exosomal PD-L1 contributes to immunosuppression and is associated with anti-PD-1 response. Nature. 2018;560:382–6. https://doi.org/10.1038/s41586-018-0392-8.
Goldvaser H, Gutkin A, Beery E, Edel Y, Nordenberg J, Wolach O, et al. Characterisation of blood-derived exosomal hTERT mRNA secretion in cancer patients: a potential pan-cancer marker. Br J Cancer. 2017;117:353–7. https://doi.org/10.1038/bjc.2017.166.
Gutkin A, Uziel O, Beery E, Nordenberg J, Pinchasi M, Goldvaser H, et al. Tumor cells derived exosomes contain hTERT mRNA and transform nonmalignant fibroblasts into telomerase positive cells. Oncotarget. 2016;7:59173–88. https://doi.org/10.18632/oncotarget.10384.
Corcoran C, Rani S, O’Driscoll L. miR-34a is an intracellular and exosomal predictive biomarker for response to docetaxel with clinical relevance to prostate cancer progression. Prostate. 2014. https://doi.org/10.1002/pros.22848.
Ayob AZ, Ramasamy TS. Cancer stem cells as key drivers of tumour progression. J Biomed Sci. 2018;25:20. https://doi.org/10.1186/s12929-018-0426-4.
Gradilla A-C, González E, Seijo I, Andrés G, Bischoff M, González-Mendez L, et al. Exosomes as Hedgehog carriers in cytoneme-mediated transport and secretion. Nat Commun. 2014;5:5649. https://doi.org/10.1038/ncomms6649.
Luga V, Zhang L, Viloria-Petit AM, Ogunjimi AA, Inanlou MR, Chiu E, et al. Exosomes mediate stromal mobilization of autocrine Wnt-PCP signaling in breast cancer cell migration. Cell. 2012;151:1542–56. https://doi.org/10.1016/j.cell.2012.11.024.
Chairoungdua A, Smith DL, Pochard P, Hull M, Caplan MJ. Exosome release of β-catenin: a novel mechanism that antagonizes Wnt signaling. J Cell Biol. 2010;190:1079–91. https://doi.org/10.1083/jcb.201002049.
Levchenko A, Mehta BM, Niu X, Kang G, Villafania L, Way D, et al. Intercellular transfer of P-glycoprotein mediates acquired multidrug resistance in tumor cells. Proc Natl Acad Sci U S A. 2005;102:1933–8. https://doi.org/10.1073/pnas.0401851102.
Pasquier J, Galas L, Boulangé-Lecomte C, Rioult D, Bultelle F, Magal P, et al. Different modalities of intercellular membrane exchanges mediate cell-to-cell p-glycoprotein transfers in MCF-7 breast cancer cells. J Biol Chem. 2012;287:7374–87. https://doi.org/10.1074/jbc.M111.312157.
Corcoran C, Rani S, O’Brien K, O’Neill A, Prencipe M, Sheikh R, et al. Docetaxel-resistance in prostate cancer: evaluating associated phenotypic changes and potential for resistance transfer via exosomes. PLoS ONE. 2012;7:e50999. https://doi.org/10.1371/journal.pone.0050999.
Ciravolo V, Huber V, Ghedini GC, Venturelli E, Bianchi F, Campiglio M, et al. Potential role of HER2-overexpressing exosomes in countering trastuzumab-based therapy. J Cell Physiol. 2012;227:658–67. https://doi.org/10.1002/jcp.22773.
Dutta S, Reamtong O, Panvongsa W, Kitdumrongthum S, Janpipatkul K, Sangvanich P, et al. Proteomics profiling of cholangiocarcinoma exosomes: a potential role of oncogenic protein transferring in cancer progression. Biochim Biophys Acta. 2015;1852:1989–99. https://doi.org/10.1016/j.bbadis.2015.06.024.
Chen W-X, Liu X-M, Lv M-M, Chen L, Zhao J-H, Zhong S-L, et al. Exosomes from drug-resistant breast cancer cells transmit chemoresistance by a horizontal transfer of microRNAs. PLoS ONE. 2014;9:e95240. https://doi.org/10.1371/journal.pone.0095240.
Zhong S, Li W, Chen Z, Xu J, Zhao J. MiR-222 and miR-29a contribute to the drug-resistance of breast cancer cells. Gene. 2013;531:8–14. https://doi.org/10.1016/j.gene.2013.08.062.
Yu D-D, Wu Y, Zhang X-H, Lv M-M, Chen W-X, Chen X, et al. Exosomes from adriamycin-resistant breast cancer cells transmit drug resistance partly by delivering miR-222. Tumour Biol. 2016;37:3227–35. https://doi.org/10.1007/s13277-015-4161-0.
Haney MJ, Zhao Y, Jin YS, Li SM, Bago JR, Klyachko NL, et al. Macrophage-derived extracellular vesicles as drug delivery systems for triple negative breast cancer (TNBC) therapy. J Neuroimmune Pharmacol. 2020. https://doi.org/10.1007/s11481-019-09884-9.
Hood JL, San RS, Wickline SA. Exosomes released by melanoma cells prepare sentinel lymph nodes for tumor metastasis. Can Res. 2011. https://doi.org/10.1158/0008-5472.can-10-4455.
Zhao C, Wang H, Xiong C, Liu Y. Hypoxic glioblastoma release exosomal VEGF-A induce the permeability of blood-brain barrier. Biochem Biophys Res Commun. 2018. https://doi.org/10.1016/j.bbrc.2018.05.140.
Pascucci L, Coccè V, Bonomi A, Ami D, Ceccarelli P, Ciusani E, et al. Paclitaxel is incorporated by mesenchymal stromal cells and released in exosomes that inhibit in vitro tumor growth: A new approach for drug delivery. J Control Release. 2014. https://doi.org/10.1016/j.jconrel.2014.07.042.
Coccè V, Franzè S, Brini A, Giannì A, Pascucci L, Ciusani E, et al. In vitro anticancer activity of extracellular vesicles (EVs) secreted by gingival mesenchymal stromal cells primed with paclitaxel. Pharmaceutics. 2019. https://doi.org/10.3390/pharmaceutics11020061.
Tian Y, Li S, Song J, Ji T, Zhu M, Anderson GJ, et al. A doxorubicin delivery platform using engineered natural membrane vesicle exosomes for targeted tumor therapy. Biomaterials. 2014;35:2383–90. https://doi.org/10.1016/j.biomaterials.2013.11.083.
Rivoltini L, Chiodoni C, Squarcina P, Tortoreto M, Villa A, Vergani B, et al. TNF-related apoptosis-inducing ligand (TRAIL)–armed exosomes deliver proapoptotic signals to tumor site. Clin Cancer Res. 2016. https://doi.org/10.1158/1078-0432.ccr-15-2170.
Wall N, Aspe R. Survivin-T34A: molecular mechanism and therapeutic potential. OncoTarget Ther. 2010. https://doi.org/10.2147/ott.s15293.
Moroishi T, Hayashi T, Pan W-W, Fujita Y, Holt MV, Qin J, et al. The hippo pathway kinases LATS1/2 suppress cancer immunity. Cell. 2016;167:1525–39. https://doi.org/10.1016/j.cell.2016.11.005.
Lou G, Song X, Yang F, Wu S, Wang J, Chen Z, et al. Exosomes derived from miR-122-modified adipose tissue-derived MSCs increase chemosensitivity of hepatocellular carcinoma. J Hematol Oncol. 2015;8:1–11. https://doi.org/10.1186/s13045-015-0220-7.
Zhang H, Wang Y, Bai M, Wang J, Zhu K, Liu R, et al. Exosomes serve as nanoparticles to suppress tumor growth and angiogenesis in gastric cancer by delivering hepatocyte growth factor siRNA. Cancer Sci. 2018. https://doi.org/10.1111/cas.13488.
Shtam TA, Kovalev RA, Varfolomeeva EY, Makarov EM, Kil YV, Filatov MV. Exosomes are natural carriers of exogenous siRNA to human cells in vitro. Cell Commun Signal. 2013;11:1–10. https://doi.org/10.1186/1478-811X-11-88.
Ohno S-I, Takanashi M, Sudo K, Ueda S, Ishikawa A, Matsuyama N, et al. Systemically injected exosomes targeted to EGFR deliver antitumor MicroRNA to breast cancer cells. Mol Ther. 2013. https://doi.org/10.1038/mt.2012.180.
Bu N, Li Q-L, Feng Q, Sun B-Z. Immune protection effect of exosomes against attack of L1210 tumor cells. Leuk Lymphoma. 2006;47:913–8. https://doi.org/10.1080/10428190500376191.
Lee E-Y, Park K-S, Yoon YJ, Lee J, Moon H-G, Jang SC, et al. Therapeutic effects of autologous tumor-derived nanovesicles on melanoma growth and metastasis. PLoS ONE. 2012;7:e33330. https://doi.org/10.1371/journal.pone.0033330.
Gehrmann U, Hiltbrunner S, Georgoudaki A-M, Karlsson MC, Näslund TI, Gabrielsson S. Synergistic induction of adaptive antitumor immunity by codelivery of antigen with α-galactosylceramide on exosomes. Cancer Res. 2013;73:3865–76. https://doi.org/10.1158/0008-5472.CAN-12-3918.
Xie Y, Bai O, Zhang H, Yuan J, Zong S, Chibbar R, et al. Membrane-bound HSP70-engineered myeloma cell-derived exosomes stimulate more efficient CD8 CTL- and NK-mediated antitumour immunity than exosomes released from heat-shocked tumour cells expressing cytoplasmic HSP70. J Cell Mol Med. 2010. https://doi.org/10.1111/j.1582-4934.2009.00851.x.
Mahmoodzadeh Hosseini H, Imani Fooladi AA, Soleimanirad J, Nourani MR, Davaran S, Mahdavi M. Staphylococcal entorotoxin B anchored exosome induces apoptosis in negative esterogen receptor breast cancer cells. Tumour Biol. 2014;35:3699–707. https://doi.org/10.1007/s13277-013-1489-1.
Escudier B, Dorval T, Chaput N, André F, Caby M-P, Novault S, et al. Vaccination of metastatic melanoma patients with autologous dendritic cell (DC) derived-exosomes: results of thefirst phase I clinical trial. J Transl Med. 2005;3:10. https://doi.org/10.1186/1479-5876-3-10.
Morse MA, Garst J, Osada T, Khan S, Hobeika A, Clay TM, et al. A phase I study of dexosome immunotherapy in patients with advanced non-small cell lung cancer. J Transl Med. 2005;3:9. https://doi.org/10.1186/1479-5876-3-9.
Besse B, Charrier M, Lapierre V, Dansin E, Lantz O, Planchard D, et al. Dendritic cell-derived exosomes as maintenance immunotherapy after first line chemotherapy in NSCLC. Oncoimmunology. 2016;5:e1071008. https://doi.org/10.1080/2162402X.2015.1071008.
Wong C-H, Chen Y-C. Clinical significance of exosomes as potential biomarkers in cancer. World J Clin Cases. 2019. https://doi.org/10.12998/wjcc.v7.i2.171.
Wang J-C, Begin LR, Berube NG, Chevalier S, Aprikian AG, Gourdeau H, et al. Down-regulation of CD9 expression during prostate carcinoma progression is associated with CD9 mRNA modifications. Clin Cancer Res. 2007. https://doi.org/10.1158/1078-0432.ccr-06-1692.
Jang H-I, Lee H. A decrease in the expression of CD63 tetraspanin protein elevates invasive potential of human melanoma cells. Exp Mol Med. 2003;35:317–23. https://doi.org/10.1038/emm.2003.43.
Sandfeld-Paulsen B, Jakobsen KR, Bæk R, Folkersen BH, Rasmussen TR, Meldgaard P, et al. Exosomal proteins as diagnostic biomarkers in lung cancer. J Thorac Oncol. 2016. https://doi.org/10.1016/j.jtho.2016.05.034.
Jin H, Liu P, Wu Y, Meng X, Wu M, Han J, et al. Exosomal zinc transporter ZIP4 promotes cancer growth and is a novel diagnostic biomarker for pancreatic cancer. Cancer Sci. 2018;109:2946–56. https://doi.org/10.1111/cas.13737.
Kimura H, Yamamoto H, Harada T, Fumoto K, Osugi Y, Sada R, et al. CKAP4, a DKK1 receptor, is a biomarker in exosomes derived from pancreatic cancer and a molecular target for therapy. Clin Cancer Res. 2019;25:1936–47. https://doi.org/10.1158/1078-0432.CCR-18-2124.
San Lucas FA, Allenson K, Bernard V, Castillo J, Kim DU, Ellis K, et al. Minimally invasive genomic and transcriptomic profiling of visceral cancers by next-generation sequencing of circulating exosomes. Ann Oncol. 2016;27:635–41. https://doi.org/10.1093/annonc/mdv604.
Bernard V, Kim DU, San Lucas FA, Castillo J, Allenson K, Mulu FC, et al. Circulating nucleic acids are associated with outcomes of patients with pancreatic cancer. Gastroenterology. 2019;156(108–118):e4. https://doi.org/10.1053/j.gastro.2018.09.022.
Pigati L, Yaddanapudi SCS, Iyengar R, Kim D-J, Hearn SA, Danforth D, et al. Selective release of MicroRNA species from normal and malignant mammary epithelial cells. PLoS ONE. 2010. https://doi.org/10.1371/journal.pone.0013515.
Sohn W, Kim J, Kang SH, Yang SR, Cho J-Y, Cho HC, et al. Serum exosomal microRNAs as novel biomarkers for hepatocellular carcinoma. Exp Mol Med. 2015;47:e184. https://doi.org/10.1038/emm.2015.68.
Yan S, Jiang Y, Liang C, Cheng M, Jin C, Duan Q, et al. Exosomal miR-6803-5p as potential diagnostic and prognostic marker in colorectal cancer. J Cell Biochem. 2018;119:4113–9. https://doi.org/10.1002/jcb.26609.
Feng Y, Zhong M, Zeng S, Wang L, Liu P, Xiao X, et al. Exosome-derived miRNAs as predictive biomarkers for diffuse large B-cell lymphoma chemotherapy resistance. Epigenomics. 2019;11:35–51. https://doi.org/10.2217/epi-2018-0123.
Pan D, Chen J, Feng C, Wu W, Wang Y, Tong J, et al. Preferential localization of MUC1 glycoprotein in exosomes secreted by non-small cell lung carcinoma cells. Int J Mol Sci. 2019. https://doi.org/10.3390/ijms20020323.
Skotland T, Ekroos K, Kauhanen D, Simolin H, Seierstad T, Berge V, et al. Molecular lipid species in urinary exosomes as potential prostate cancer biomarkers. Eur J Cancer. 2017;70:122–32. https://doi.org/10.1016/j.ejca.2016.10.011.
Re MD, Del Re M, Marconcini R, Pasquini G, Rofi E, Vivaldi C, et al. PD-L1 mRNA expression in plasma-derived exosomes is associated with response to anti-PD-1 antibodies in melanoma and NSCLC. Br J Cancer. 2018. https://doi.org/10.1038/bjc.2018.9.
Saari H, Lázaro-Ibáñez E, Viitala T, Vuorimaa-Laukkanen E, Siljander P, Yliperttula M. Microvesicle- and exosome-mediated drug delivery enhances the cytotoxicity of Paclitaxel in autologous prostate cancer cells. J Control Release. 2015;220:727–37. https://doi.org/10.1016/j.jconrel.2015.09.031.
Melzer C, Rehn V, Yang Y, Bähre H, von der Ohe J, Hass R. Taxol-loaded MSC-derived exosomes provide a therapeutic vehicle to target metastatic breast cancer and other carcinoma cells. Cancers (Basel). 2019. https://doi.org/10.3390/cancers11060798.
Agrawal AK, Aqil F, Jeyabalan J, Spencer WA, Beck J, Gachuki BW, et al. Milk-derived exosomes for oral delivery of paclitaxel. Nanomedicine. 2017;13:1627–36. https://doi.org/10.1016/j.nano.2017.03.001.
Kim MS, Haney MJ, Zhao Y, Yuan D, Deygen I, Klyachko NL, et al. Engineering macrophage-derived exosomes for targeted paclitaxel delivery to pulmonary metastases: in vitro and in vivo evaluations. Nanomedicine. 2018;14:195–204. https://doi.org/10.1016/j.nano.2017.09.011.
Gomari H, Forouzandeh Moghadam M, Soleimani M. Targeted cancer therapy using engineered exosome as a natural drug delivery vehicle. Onco Targets Ther. 2018;11:5753–62. https://doi.org/10.2147/OTT.S173110.
Xie Y, Bai O, Zhang H, Yuan J, Zong S, Chibbar R, et al. Membrane-bound HSP70-engineered myeloma cell-derived exosomes stimulate more efficient CD8(+) CTL- and NK-mediated antitumour immunity than exosomes released from heat-shocked tumour cells expressing cytoplasmic HSP70. J Cell Mol Med. 2010;14:2655–66. https://doi.org/10.1111/j.1582-4934.2009.00851.x.
Wang J-C, Bégin LR, Bérubé NG, Chevalier S, Aprikian AG, Gourdeau H, et al. Down-regulation of CD9 expression during prostate carcinoma progression is associated with CD9 mRNA modifications. Clin Cancer Res. 2007;13:2354–61. https://doi.org/10.1158/1078-0432.CCR-06-1692.
Acknowledgment
The authors would also like to express their most sincere words of appreciation to Dr. Elham Jamshidi for her valuable contribution.
Funding
This study is related to project NO 1398/4083 From the Student Research Committee, Shahid Beheshti University of Medical Sciences, Tehran, Iran. We also appreciate the "Student Research Committee" and "Research & Technology Chancellor" at Shahid Beheshti University of Medical Sciences for their financial support of this study.
Author information
Authors and Affiliations
Contributions
Conceptualization: AJ, AB, NA, MRT and MA; investigation: AJ, AB, MA, NA; writing—original draft preparation: AJ, AB; writing—review and editing: AJ, AB, and MA; supervision: MRT. All authors read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Informed consent
The Authors grant the Publisher the sole and exclusive license of the full copyright in the Contribution, which licenses the Publisher hereby accepts. Consequently, the Publisher shall have the exclusive right to publish and sell the Contribution in all languages throughout the world.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Jafari, A., Babajani, A., Abdollahpour-Alitappeh, M. et al. Exosomes and cancer: from molecular mechanisms to clinical applications. Med Oncol 38, 45 (2021). https://doi.org/10.1007/s12032-021-01491-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12032-021-01491-0