Abstract
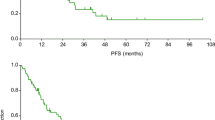
Clinical practice shows significant differences in treatment outcomes across patients treated with cabozantinib for metastatic renal cell carcinoma (mRCC). It is not known whether cabozantinib-induced adverse events are predictive factors of survival as in case of drugs such as sunitinib or axitinib. The study participants were 30 adult patients with mRCC treated with cabozantinib as a second- or further line setting. All adverse events were evaluated using the National Cancer Institute Common Terminology Criteria for Adverse Events version 4.0. Progression-free survival (PFS) values were calculated by taking the beginning of cabozantinib treatment as the start date and either disease progression or death as the end date. PFS were estimated using the Kaplan–Meier method, and compared using the log-rank test. We identified independent PFS predictors using multiple Cox proportional hazards models and reported hazard ratios (HR) with 95% confidence intervals. The median observation time cabozantinib treatment was 7.5 months, with a range of 2–15 months. During that time, 11 (37%) of the patients had mRCC progression. Median PFS on cabozantinib was not reached, and lower quartile was 6 months. All patients developed at least one adverse event in the course of cabozantinib therapy. Hypertension, hypothyroidism and HFS were observed most frequently, in about two-thirds of the patients. The co-incidence of multiple adverse events was common. Hypertension, hypothyroidism, diarrhea and liver toxicity were significantly associated with longer PFS values. Patients with three or more side effects had significantly longer PFS than those with two or fewer. Even though this study was conducted in a small patient sample and the observation time was relatively short our results confirm the predictive value of the incidence of adverse events during cabozantinib treatment in mRCC patients. To the best of our knowledge, this is the first study of this kind conducted in this group of patients.

Similar content being viewed by others
References
Motzer RJ, Escudier B, McDermott DF, George S, Hammers HJ, Srinivas S, Tykodi SS, Sosman JA, Procopio G, Plimack ER, Castellano D, Choueiri TK, Gurney H, Donskov F, Bono P, Wagstaff J, Gauler TC, Ueda T, Tomita Y, Schutz FA, Kollmannsberger C, Larkin J, et al. Nivolumab versus everolimus in advanced renal-cell carcinoma. N Engl J Med. 2015;373(19):1803–13. https://doi.org/10.1056/NEJMoa1510665.
Motzer RJ, Tannir NM, McDermott DF, Arén Frontera O, Melichar B, Choueiri TK, Plimack ER, Barthélémy P, Porta C, George S, Powles T, Donskov F, Neiman V, Kollmannsberger CK, Salman P, Gurney H, Hawkins R, Ravaud A, Grimm MO, Bracarda S, Barrios CH, Tomita Y, et al. Nivolumab plus Ipilimumab versus sunitinib in advanced renal-cell carcinoma. N Engl J Med. 2018;378(14):1277–90. https://doi.org/10.1056/NEJMoa1712126.
Yakes FM, Chen J, Tan J, et al. Cabozantinib (XL184), a novel MET and VEGFR2 inhibitor, simultaneously suppresses metastasis,angiogenesis, and tumor growth. Mol Cancer Ther. 2011;10:2298–308.
Zhou L, Liu XD, Sun M, et al. Targeting MET and AXL overcomes resistance to sunitinib therapy in renal cell carcinoma. Oncogene. https://doi.org/10.1038/onc.2015.343.
Choueiri TK, Escudier B, Powles T, Tannir NM, Mainwaring PN, Rini BI, Hammers HJ, Donskov F, Roth BJ, Peltola K, Lee JL, Heng DYC, Schmidinger M, Agarwal N, Sternberg CN, McDermott DF, Aftab DT, Hessel C, Scheffold C, Schwab G, Hutson TE, Pal S, et al. Cabozantinib versus everolimus in advanced renal cell carcinoma (METEOR): final results from a randomised, open-label, phase 3 trial. Lancet Oncol. 2016;17(7):917–27. https://doi.org/10.1016/S1470-2045(16)30107-3.
Ravaud A, Schmidinger M. Clinical biomarkers of response in advanced renal cell carcinoma. Ann Oncol. 2013;24(12):2935–42.
Rini BI, Tamaskar I, Shaheen P, Salas R, Garcia J, Wood L, et al. Hypothyroidism in patients with metastatic renal cell carcinoma treated with sunitinib. J Natl Cancer Inst. 2007;99(1):81–3.
Rini BI, Cohen DP, Lu DR, Chen I, Hariharan S, Gore ME, et al. Hypertension as biomarker of efficacy in patients with metastatic renal cell carcinoma treated with sunitinib. J Natl Cancer Inst. 2011;103(9):763–73.
Buda-Nowak A, Kucharz J, Dumnicka P, Kuzniewski M, Herman RM, Zygulska AL, Kusnierz-Cabala B. Sunitinib-induced hypothyroidism predicts progression-free survival in metastatic renal cell carcinoma patients. Med Oncol. 2017;34(4):68. https://doi.org/10.1007/s12032-017-0928-z.
Pusanov I, Michaelson D, Cohen D, et al. Evaluation of hand-foot syndrome (HFS) as a potential biomarker of sunitinib efficacy in patients with metastatic renal cell carcinoma (mRCC) and gastrointestinal stromal tumour (GIST). ECCO16-ESMO36- ESTRO30 2011 (Abstract 1444).
Rini BI, Garrett M, Poland B, et al. Axitinib in metastatic renal cell carcinoma: results of a pharmacokinetic and pharmacodynamic analysis. J Clin Pharmacol. 2013;53:491–504.
Kucharz J, Dumnicka P, Kuzniewski M, Kusnierz-Cabala B, Herman RM, Krzemieniecki K. Co-occurring adverse events enable early prediction of progression-free survival in metastatic renal cell carcinoma patients treated with sunitinib: a hypothesis-generating study. Tumori J. 2015;101:555–9. https://doi.org/10.5301/tj.5000342.
Nagyiványi K, Budai B, Bíró K, Gyergyay F, Noszek L, Küronya Z, Németh H, Nagy P, Géczi L. Synergistic survival: a new phenomenon connected to adverse events of first-line sunitinib treatment in advanced renal cell carcinoma. Clin Genitourin Cancer. 2016;14(4):314–22. https://doi.org/10.1016/j.clgc.2015.11.016.
Huh CG, Factor VM, Sánchez A, Uchida K, Conner EA, Thorgeirsson SS. Hepatocyte growth factor/c-met signaling pathway is required for efficient liver regeneration and repair. Proc Natl Acad Sci USA. 2004;101(13):4477–82.
Giebeler A, Boekschoten MV, Klein C. et al. c-Met confers protection against chronic liver tissue damage and fibrosis progression after bile duct ligation in mice. Gastroenterology. 2009;137(1):297–308.
Marquardt JU, Seo D, Gómez-Quiroz LE. et al. Loss of c-Met accelerates development of liver fibrosis in response to CCl(4) exposure through deregulation of multiple molecular pathways. Biochim Biophys Acta. 2012;1822(6):942–51.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Jakub Kucharz has received research grant from Novartis, travel grants and speakers’ honoraria from Pfizer, Bayer, Novartis and IPSEN. Pawel Wiechno has received speakers’ honoraria and travel Grants from Pfizer, Bayer, Novartis and IPSEN. Other authors declare no conflicts of interest.
Rights and permissions
About this article
Cite this article
Kucharz, J., Dumnicka, P., Kusnierz-Cabala, B. et al. The correlation between the incidence of adverse events and progression-free survival in patients treated with cabozantinib for metastatic renal cell carcinoma (mRCC). Med Oncol 36, 19 (2019). https://doi.org/10.1007/s12032-018-1239-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12032-018-1239-8