Abstract
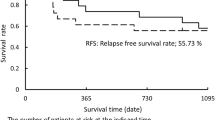
The aim of the study was to evaluate the efficacy and safety of intratumoral chemotherapy with paclitaxel liposome combined with systemic chemotherapy as induction therapy in clinical stage III unresectable non-small cell lung cancer (NSCLC). Between January 2011 and July 2014, 48 patients, stage III, performance status 0–1, with unresectable clinical stage IIIA or IIIB NSCLC suitable for definitive radiation treatment, were included in the study. Patients with T3N1M0 and T4 (ipsilateral lung nodules) N0-1M0 were not included. Patients were given 3 cycles of chemotherapy every 3 weeks. Carboplatin (AUC5 by i.v. on day 1) and gemcitabine (i.v. 1,000 mg/m2 on days 1 and 8) were administered. Paclitaxel liposome was injected at 1–3 mg/ml concentration into the tumor lesion by computed tomography-guided percutaneous fine-needle intratumoral injection and proven malignant lymph nodes according to pretreatment histological/cytological results by endobronchial ultrasound drug delivery with a needle on day 1 and day 8. Toxicity was assessed on days 8 and 22 in each cycle. Overall response rate (ORR) evaluation was performed at the end of cycle 3. Out of the 48 enrolled patients, 28 were males and 20 females, 19 patients had stage IIIA and 29 stage IIIB NSCLC. Thirty-six partial responses and two complete responses were observed, for an ORR of 81 %. The most frequent G3–G4 toxicity included neutropenia (in 15 % of cases), hypertransaminasemia (6 %), and diarrhea (4 %). A median PFS of 16.5 months (95 % CI 13.7–19.2) and median OS of 23.2 months (95 % CI 20.0–26.3) were observed. Eleven stage IIIA patients underwent surgery, for a resection rate of 58 %. Intratumoral chemotherapy with paclitaxel liposome combined with systemic chemotherapy demonstrated a considerable disease response and resection rate, with acceptable toxicity.



Similar content being viewed by others
References
Yang P, Allen MS, Aubry MC, Wampfler JA, Marks RS, Edell ES, et al. Clinical features of 5,628 primary lung cancer patients: experience at Mayo Clinic from 1997–2003. Chest. 2005;128(1):452–62.
Govindan R, Page N, Morgensztern D, Read W, Tierney R, Vlahiotis A, et al. Changing epidemiology of small-cell lung cancer in the United States over the last 30 years: analysis of the surveillance, epidemiologic, and end results database. J Clin Oncol. 2006;24:4539–44.
Pfister DG, Johnson DH, Azzoli CG, et al. American Society of Clinical Oncology treatment of unresectable non-small-cell lung cancer guidelines: update 2003. J Clin Oncol. 2004;22:330–53.
Furuse K, Fukuoka M, Kawakara M, et al. Phase III study of concurrent versus sequential thoracic radiotherapy in combination with mitomycin, vindesine and cisplatin in unresectable stage III non-small-cell lung cancer. J Clin Oncol. 1999;17:2692–9.
Curran WJ Jr, Paulus R, Langer CJ, et al. Sequential vs. concurrent chemoradiation for stage III non-small cell lung cancer: randomized phase III trial RTOG 9410. J Natl Cancer Inst. 2011;103:1452–60.
Fournel P, Robinet G, Thomas P, et al. Randomized phase III trial of sequential chemoradiotherapy compared with concurrent chemoradiotherapy in locally advanced non-small-cell lung cancer: groupe Lyon-Saint-Etienne d’Oncologie Thoracique-Groupe Franc¸ais de Pneumo-Cance´rologie NPC 95–01 Study. J Clin Oncol. 2005;23:5910–7.
Zatloukal P, Petruzelka I, Zemanova M, et al. Concurrent versus sequential chemoradiotherapy with cisplatin and vinorelbine in locally advanced non-small cell lung cancer: a randomized study. Lung Cancer. 2004;46:87–98.
Rowell NP, O’rourke NP. Concurrent chemoradiotherapy in nonsmall cell lung cancer. Cochrane Database Syst Rev. 2004;18:CD002140.
Gandara DR, Chansky K, Albain KS, et al. Long-term survival with concurrent chemoradiation therapy followed by consolidation docetaxel in stage IIIB non-small-cell lung cancer: a phase II Southwest Oncology Group Study (S9504). Clin Lung Cancer. 2006;8:116–21.
Curran WJ, Scott CB, Langer CJ, et al. Long-term benefit is observed in a phase III comparison of sequential vs concurrent chemo-radiation for patients with unresected stage III nsclc: RTOG 9410 (Abstract). Proc Am Soc Clin Oncol. 2003;22:2499.
Schaake-Koning C, van den Bogaert W, Dalesio O, et al. Effects of concomitant cisplatin and radiotherapy on inoperable non-small-cell lung cancer. N Engl J Med. 1992;326:524–30.
Vokes EE, Herndon JE, Kelley MJ, Watson D, Cicchetti MG, Green MR. Induction chemotherapy followed by concomitant chemoradiotherapy (CT/XRT) versus CT/XRT alone for regionally advanced unresect-able non-small cell lung cancer (NSCLC): initial analysis of a randomized phase III trial (Abstract). Proc Am Soc Clin Oncol. 2004;22(14S):7005.
Vokes EE, Herndon JE II, Crawford J, et al. Randomized phase II study of cisplatin with gemcitabine or paclitaxel or vinorelbine as induction chemotherapy followed by concomitant chemoradiotherapy for stage IIIB non-small-cell lung cancer: cancer and leukemia group B study 9431. J Clin Oncol. 2002;20:4191–8.
Socinski MA, Rosenman JG, Halle J, et al. Dose-escalating conformal thoracic radiation therapy with induction and concurrent carboplatin/paclitaxel in unresectable stage IIIA/B nonsmall cell lung carcinoma: a modified phase I/II trial. Cancer. 2001;92:1213–23.
Faber LP, Kittle CF, Warren WH, et al. Preoperative chemotherapy and irradiation for stage III non-small-cell lung cancer. Ann Thorac Surg. 1989;47:669–75 discussion, 76–77.
Cohen EE, Vokes EE. Induction chemotherapy and radiotherapy in locally advanced non-small-cell lung cancer. Hematol Oncol Clin N Am. 2004;18:81–90.
Paccagnella A, Oniga F, Bearz A, et al. Adding gemcitabine to paclitaxel/carboplatin increases survival in advanced non-smallcell lung cancer: results of a phase II–III study. J Clin Oncol. 2006;24:681–7.
Abratt RP, Lee JS, Han JY, et al. Phase II trial of gemcitabine, carboplatin–paclitaxel as neoadjuvant chemotherapy for operablenon-small-cell lung cancer. J Thorac Oncol. 2006;1:135–40.
Schallier D, Neyns B, Fontaine CA, et al. A novel triplet regimen with paclitaxel, carboplatin and gemcitabine (PACCAGE) as induction chemotherapy for locally advanced unresectable non small cell lung cancer (NSCLC). Lung Cancer. 2007;56:247–54.
McLaughlin CA, Goldberg EP. Targeted drugs. In: Goldberg EP, editor Local chemo- and immunotherapy by intratumor drug injection: opportunities for polymer drug compositions. NY: Wiley Interscience; 1983. p. 231–68 [Chapter 10].
Goldberg EP, Hadba AR, Almond BA, Marotta JS. Intratumoral cancer chemotherapy and immunotherapy: opportunities for non-systemic preoperative drug delivery. J Pharm Pharmacol. 2002;54:159–80.
Celikoglu F, Celikoglu SI, York AM, Goldberg EP. Intratumoral administration of cisplatin through a bronchoscope followed by irradiation for treatment of inoperable non-small cell obstructive lung cancer. Lung Cancer. 2006;51:225–36.
Celikoglu SI, Celikoglu F, Goldberg EP. Endobronchial intratumoral chemotherapy (EITC) followed by surgery in early non-small-cell lung cancer with polypoid growth causing erroneous impression of advanced disease. Lung Cancer. 2006;54:339–46.
Mountain CF. Revisions in the international system for staging lung cancer. Chest. 1997;111:1710–7.
Ratain MJ, Eckhardt SG. Phase II studies of modern drugs directed against new targets: if you are fazed, too, then resist RECIST. J Clin Oncol. 2004;22:4442–5.
Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–81.
Vinh-Hung V. Power of test comparing independent proportions. Comput Biol Med. 1986;16:39–43.
Bunn PA Jr. Chemotherapy for advanced non-small-cell lung cancer: who, what, where, why? J Clin Oncol. 2002;20(18 Suppl):23S–33S.
Lammers T, et al. Effect of intratumoral injection on the biodistribution and the therapeutic potential of HPMA copolymer-based drug delivery systems. Neoplasia. 2006;8:788–95.
Banna GL, et al. A three-drug induction chemotherapy with gemcitabine, carboplatin, and paclitaxel for stage III non-small cell lung cancer. Med Oncol. 2013;30:533. doi:10.1007/s12032-013-0533-8.
Schallier D, Bral S, Ilsen B, Neyns B, Fontaine C, Decoster L, De Mey J, Meysman M, De Greve J. Final overall results of a study with a novel triplet induction chemotherapy regimen (PACCAGE) followed by consolidation radiotherapy in locally advanced inoperable non-small cell lung cancer (NSCLC). J Thorac Oncol. 2009;4:728–35.
Hohenforst-Schmidt W. Intratumoral Chemotherapy (ITC): a forgotten option at least in functionally or oncologically driven palliation! Paper presented at: 16th WCBE 20102010.
Celikoglu F, Celikoglu SI, Goldberg EP. Bronchoscopic intratumoral chemotherapy of lung cancer. Lung Cancer. 2008;61(1):1–12.
Conflict of interest
All authors have no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lu, B., Sun, L., Yan, X. et al. Intratumoral chemotherapy with paclitaxel liposome combined with systemic chemotherapy: a new method of neoadjuvant chemotherapy for stage III unresectable non-small cell lung cancer. Med Oncol 32, 345 (2015). https://doi.org/10.1007/s12032-014-0345-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12032-014-0345-5